Sandbox 171
From Proteopedia
| Line 49: | Line 49: | ||
''' | ''' | ||
''' | ''' | ||
| - | [[Image:Example.jpg]] | ||
Revision as of 05:55, 1 April 2010
| Please do NOT make changes to this Sandbox until after April 23, 2010. Sandboxes 151-200 are reserved until then for use by the Chemistry 307 class at UNBC taught by Prof. Andrea Gorrell. | |||||||||
| |||||||||
| 2mys, resolution 2.80Å () | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Ligands: | , | ||||||||
| Non-Standard Residues: | |||||||||
| |||||||||
| |||||||||
| Resources: | FirstGlance, OCA, PDBsum, RCSB | ||||||||
| Coordinates: | save as pdb, mmCIF, xml | ||||||||
Contents |
Overview
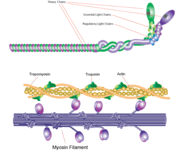
Myosin is one of three major classes of molecular motor proteins: myosin, dynein, and kinesin. As the most abundant of these proteins myosin plays a structural and enzymatic role in muscle contraction and intracellular motility. Myosin was first discovered in muscle in the 19th century. [1]
Crystallization and X-ray diffraction
Myosin is found in abundance, therefore it can be prepared in gram quantities. [2] For nearly 30 years the myosin head was resistant to crystallization, yet by 1993 researchers discovered a mechanism to obtain x-ray quality crystals. [2] The process modified the protein by reductive methylation. [2] X-ray data was used to determine the tertiary structure of the protein. [2]
Structure
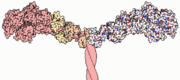
Myosin has a molecular size of approximately 520 kilodaltons with a total of six subunits. It has two 220 kD heavy chains which make the majority of the overall structure and two pairs of light chains which vary in size.[2] The molecule is asymmetric, having a long tail and two globular heads. [2] Each heavy chains composes the bulk of one of the globular heads. [2] Subfragment-1(S1) also termed the myosin head consists of ATP, actin, and two light chain binding sites.[2] Each globular head has a heavy chain and two light chains for a combined molecular size of about 130 kD. [2]
The myosin head is assymetrical with a length of 165 Angstroms and 65 Angstroms in width, with a total thickness of about 40 Angstroms. [2] About 48% of the amino acid residues in the myosin head are dominated by α helices. [2] At the carboxyl terminus one long α helix of about 85 Angstroms extends in a left-handed coil. [2] This particular helix forms the light chain binding region of the globular domain [2] The amino terminus of each heavy chain has a large globular domain containing the site of ATP hydrolysis.