User:Kelly Roark/Sandbox1
From Proteopedia
| Line 1: | Line 1: | ||
== Lactate Dehydrogenase == | == Lactate Dehydrogenase == | ||
| + | |||
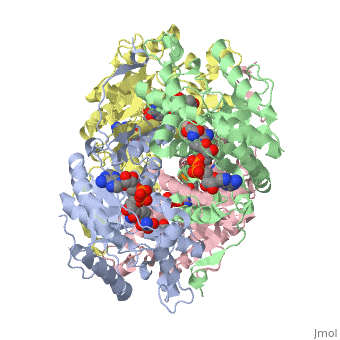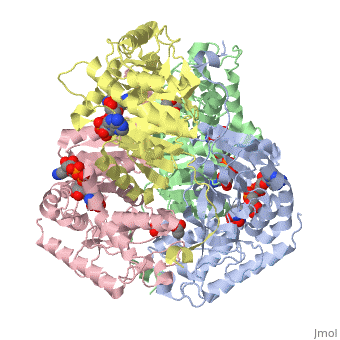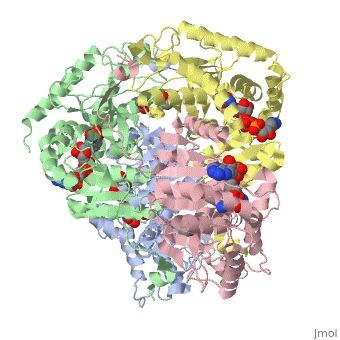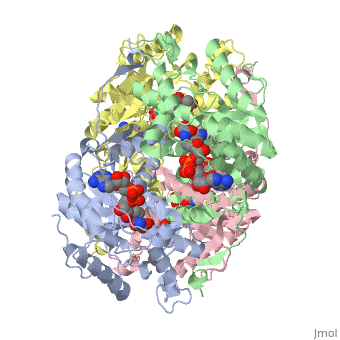
| + | <applet load='1i0z' size='300' frame='true' align='left' caption='Human heart l-lactate dehydrogenase h chain, ternary complex with nadh and oxamate' /> | ||
| + | |||
| + | <applet load='1i10' size='300' frame='true' align='right' caption='Human muscle l-lactate dehydrogenase m chain, ternary complex with nadh and oxamate'/> | ||
| + | |||
Lactate dehydrogenase (LDH) is an enzyme that catalyzes the interconversion of lactate and pyruvate (Market 1984). The direction of the reaction is determined by the relative concentration of each substrate (Doctors Lounge). Nicotinamide adenine dinucleotide (NAD+ or NADH) is the cofactor for the reaction aand acts as a hydrogen acceptor or donor (McClendon). | Lactate dehydrogenase (LDH) is an enzyme that catalyzes the interconversion of lactate and pyruvate (Market 1984). The direction of the reaction is determined by the relative concentration of each substrate (Doctors Lounge). Nicotinamide adenine dinucleotide (NAD+ or NADH) is the cofactor for the reaction aand acts as a hydrogen acceptor or donor (McClendon). | ||
| Line 11: | Line 16: | ||
LDH is purified from both animal and plant tissues based on its biochemical properties. There are twice as many negatively charged amino acids than positively charged residues with an overall negative chg at pH 7 of 69. This property can be exploited for anion exchange chromatgraphy in a initial purification step. It is also possible to use its affinity for an NAD+ analog or it substrates to purify LDH using affinity chromatography. The individual subunits have a molecular weight of around 35,000 kDa(Labrou and Clonis). The activity of the enzyme can be assayed by monitoring changes in ratio of NAD+ to NADH at 340nm on a spectrophotometer where an increase in nm means NAD+ is changing to NADH and vice versa (Market 1984). | LDH is purified from both animal and plant tissues based on its biochemical properties. There are twice as many negatively charged amino acids than positively charged residues with an overall negative chg at pH 7 of 69. This property can be exploited for anion exchange chromatgraphy in a initial purification step. It is also possible to use its affinity for an NAD+ analog or it substrates to purify LDH using affinity chromatography. The individual subunits have a molecular weight of around 35,000 kDa(Labrou and Clonis). The activity of the enzyme can be assayed by monitoring changes in ratio of NAD+ to NADH at 340nm on a spectrophotometer where an increase in nm means NAD+ is changing to NADH and vice versa (Market 1984). | ||
| - | |||
| - | <applet load='1i0z' size='300' frame='true' align='right' caption='Human heart l-lactate dehydrogenase h chain, ternary complex with nadh and oxamate' /> | ||
| - | |||
| - | <applet load='1i10' size='300' frame='true' align='right' caption='Human muscle l-lactate dehydrogenase m chain, ternary complex with nadh and oxamate'/> | ||
Revision as of 02:24, 4 April 2010
Lactate Dehydrogenase
|
|
Lactate dehydrogenase (LDH) is an enzyme that catalyzes the interconversion of lactate and pyruvate (Market 1984). The direction of the reaction is determined by the relative concentration of each substrate (Doctors Lounge). Nicotinamide adenine dinucleotide (NAD+ or NADH) is the cofactor for the reaction aand acts as a hydrogen acceptor or donor (McClendon).
LDH functions as a tetramer in vivo and is made of two kinds of subunits, H and M, each of which are encoded by a different gene. This results in 5 different isoenzymes (2 homotetramers and 3 heterotetramers) (Worthington Biochem). Different isoenzymes have different catalytic, physical properties, are regulated differently, and expressed in different kinds of tissues (Worthington) These differences are due to the different kinetic properties of the different subunits(Worthington). The subunits associate randomly; thus, isoenzyme expression in different tissues is determined by the availability of each type of subunit, which in turn is decided by the relative synthesis rates of the subunits and the gene expression for each tissue type(Market 1984)
The H subunit is more commonly found in tissues with a ready source of oxygen and that metabolize lactate including the heart and the brain. In heart muscle, the H4 homotetramer is most commonly found (LDH-1 isoenzyme) but the H3M isoenzyme is also found at a lower level of expression.
LDH-1 usually functions in the cytoplasm and is not membrane bound like the M subunit isoenzymes may be. Due to high ratio of NAD+: NADH, the H subunit in heart muscle is usually complexed with NAD+ (Simpson 1982). The H subunit form of LDH usually catalyzes the forward reaction: the oxidation of lactate to pyruvate shown above. This generates NADH which is then further utilized in the cytochrome system to generate more energy (ATP)(Market 1984). The H subunit is regulated by a negative feedback inhibition loop and is inhibited by millimolar amounts of pyruvate (Simpson 1982). When pyruvate levels reach a threshold level, it binds to and forms a complex with LDH, inactivating it. This decreases the number of available enzymes to catalyze the reaction and stalls the conversion of lactate to pyruvate and also keeps the NAD+:NADH ratio at an optimum level(Market 1984).
LDH is purified from both animal and plant tissues based on its biochemical properties. There are twice as many negatively charged amino acids than positively charged residues with an overall negative chg at pH 7 of 69. This property can be exploited for anion exchange chromatgraphy in a initial purification step. It is also possible to use its affinity for an NAD+ analog or it substrates to purify LDH using affinity chromatography. The individual subunits have a molecular weight of around 35,000 kDa(Labrou and Clonis). The activity of the enzyme can be assayed by monitoring changes in ratio of NAD+ to NADH at 340nm on a spectrophotometer where an increase in nm means NAD+ is changing to NADH and vice versa (Market 1984).