We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
Rhodopsin
From Proteopedia
(Difference between revisions)
(New page: {{STRUCTURE_1u19| PDB=1u19 | SCENE=Sandbox_173/Default_rhodopsin_pdb_1u19/1 }} ==Introduction== ===Rhodopsin=== Rhodopsin, a homodimeric protein, is a highly characterized [http://en....) |
|||
| Line 1: | Line 1: | ||
| - | + | <StructureSection load='1u19' size='500' side='right' scene='Sandbox_173/Default_rhodopsin_pdb_1u19/1'> | |
==Introduction== | ==Introduction== | ||
===Rhodopsin=== | ===Rhodopsin=== | ||
| Line 6: | Line 6: | ||
===G Protein-Coupled Receptors=== | ===G Protein-Coupled Receptors=== | ||
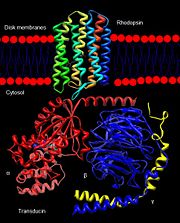
Rhodopsin is a member of the superfamily of G protein-coupled receptors that incorporate the activation of G proteins in their modulation of signaling and intracellular actions. Rhodopsin shares similar membrane topology with the members of the superfamily (Family A of the G protein-coupled receptors) which include the seven transmembrane helices, an extracellular N terminus and cytoplasmic C terminus<ref name="Article20">PMID:15251227</ref>. The seven-helical pattern is found from archaebacteria (specifically studied is bacteriorhodopsin) to humans, both which share the same retinylidene chromophore as well <ref name="Article12"/>. As the crystal structure for any G protein-coupled receptor with the seven transmembrane domain has only been solved for rhodopsin, rhodopsin may act as a reference for the structure and function relationship for other G protein-coupled receptors<ref name="Article20"/>. Like most G protein-coupled receptors, the activated rhodopsin catalyzes uptake of GTP by the heterotrimeric G protein, in this case [http://en.wikipedia.org/wiki/Transducin transducin], which interacts with the cytoplasmic loops of the receptor<ref name="Article10">PMID:11698103</ref>. However, the covalent binding nature of rhodopsin to its retinal ligand is unlike most G protein-coupled receptors. As well, another difference of rhodopsin from the members of this superfamily relates to light as the inducer for activation<ref name="Article20"/>. | Rhodopsin is a member of the superfamily of G protein-coupled receptors that incorporate the activation of G proteins in their modulation of signaling and intracellular actions. Rhodopsin shares similar membrane topology with the members of the superfamily (Family A of the G protein-coupled receptors) which include the seven transmembrane helices, an extracellular N terminus and cytoplasmic C terminus<ref name="Article20">PMID:15251227</ref>. The seven-helical pattern is found from archaebacteria (specifically studied is bacteriorhodopsin) to humans, both which share the same retinylidene chromophore as well <ref name="Article12"/>. As the crystal structure for any G protein-coupled receptor with the seven transmembrane domain has only been solved for rhodopsin, rhodopsin may act as a reference for the structure and function relationship for other G protein-coupled receptors<ref name="Article20"/>. Like most G protein-coupled receptors, the activated rhodopsin catalyzes uptake of GTP by the heterotrimeric G protein, in this case [http://en.wikipedia.org/wiki/Transducin transducin], which interacts with the cytoplasmic loops of the receptor<ref name="Article10">PMID:11698103</ref>. However, the covalent binding nature of rhodopsin to its retinal ligand is unlike most G protein-coupled receptors. As well, another difference of rhodopsin from the members of this superfamily relates to light as the inducer for activation<ref name="Article20"/>. | ||
| - | |||
==Structure== | ==Structure== | ||
| - | <applet load='1u19' size='300' color='black' frame='true' align='right' caption='Structure of Rhodopsin. The generated structures are from Chain A.'/> | ||
===Rhodopsin Architecture=== | ===Rhodopsin Architecture=== | ||
Rhodopsin consists of seven mostly α-helical transmembrane domains (H1-H7) linked sequentially by extracellular and cytoplasmic loops (E1-E3 and C1-C3 respectively), with the extracellular amino-terminal tail and the cytoplasmic carboxyl-terminal tail<ref name="Article12"/>. Four of the helices are tilted and three of the helices are approximately perpendicular to the membrane plane<ref name="Article4">PMID:9199406</ref>. There is notable interaction between the four extracellular domains, but only a few associations are observed with the cytoplasmic domains<ref name="Article9">PMID:11343925</ref>. Helix 7 is close to being elongated around the Lysine 296 retinal attachment site, and also contains the residues Proline 291 and Proline 303, with Proline 303 being part of a conserved motif<ref name="Article9"/>. Near the retinal region, there is a <scene name='Sandbox_173/Beta_4_strand_and_retinal/2'>β4 strand (Serine 186-Cysteine 187-Glycine 188-Isoleucine 189)</scene> within the Extracellular Helix 2 that runs almost parallel to the chromophore held in place and is stabilized by the essential conserved | Rhodopsin consists of seven mostly α-helical transmembrane domains (H1-H7) linked sequentially by extracellular and cytoplasmic loops (E1-E3 and C1-C3 respectively), with the extracellular amino-terminal tail and the cytoplasmic carboxyl-terminal tail<ref name="Article12"/>. Four of the helices are tilted and three of the helices are approximately perpendicular to the membrane plane<ref name="Article4">PMID:9199406</ref>. There is notable interaction between the four extracellular domains, but only a few associations are observed with the cytoplasmic domains<ref name="Article9">PMID:11343925</ref>. Helix 7 is close to being elongated around the Lysine 296 retinal attachment site, and also contains the residues Proline 291 and Proline 303, with Proline 303 being part of a conserved motif<ref name="Article9"/>. Near the retinal region, there is a <scene name='Sandbox_173/Beta_4_strand_and_retinal/2'>β4 strand (Serine 186-Cysteine 187-Glycine 188-Isoleucine 189)</scene> within the Extracellular Helix 2 that runs almost parallel to the chromophore held in place and is stabilized by the essential conserved | ||
| Line 22: | Line 20: | ||
The structure of rhodopsin may provide stability to the important Schiff base linkage with the retinal by affecting its hydrolysis, limiting its interactions with solvent, and inhibiting its release when hydrolyzed, thus encouraging rebinding of the Schiff base linkage<ref name="Article3">PMID:14611935</ref>. | The structure of rhodopsin may provide stability to the important Schiff base linkage with the retinal by affecting its hydrolysis, limiting its interactions with solvent, and inhibiting its release when hydrolyzed, thus encouraging rebinding of the Schiff base linkage<ref name="Article3">PMID:14611935</ref>. | ||
| - | |||
| - | <applet load='1u19' size='300' color='black' frame='true' align='right' caption='11-cis Retinylidene Chromophore. The generated structures are from Chain A.'/> | ||
===Retinal Chromophore of Rhodopsin=== | ===Retinal Chromophore of Rhodopsin=== | ||
| Line 30: | Line 26: | ||
As this ligand is bound in the 12-s-''trans'' conformation, there arises the non-bonding interactions between the C-13 methyl group and C-10 hydrogen that contribute to non-planarity. This leads to the ability of the chromophore polyene tail to undergo fast photoisomerization around the C-11=C-12 double bond during light-induced activation<ref name="Article2">PMID:16962138</ref>. Also, it is found that the C-11=C-12 double bond is pre-twisted in the ground state of rhodopsin, which is partly attributed to the C20 methyl group attached to C13 through interaction with Tryptophan 265. This pre-twist may give insight on the features of isomerization about this bond upon light activation<ref name="ReferenceArticle"/>. | As this ligand is bound in the 12-s-''trans'' conformation, there arises the non-bonding interactions between the C-13 methyl group and C-10 hydrogen that contribute to non-planarity. This leads to the ability of the chromophore polyene tail to undergo fast photoisomerization around the C-11=C-12 double bond during light-induced activation<ref name="Article2">PMID:16962138</ref>. Also, it is found that the C-11=C-12 double bond is pre-twisted in the ground state of rhodopsin, which is partly attributed to the C20 methyl group attached to C13 through interaction with Tryptophan 265. This pre-twist may give insight on the features of isomerization about this bond upon light activation<ref name="ReferenceArticle"/>. | ||
Somewhat enclosing this chromophore is a retinal binding pocket partially formed by the N-terminal domain overlaying the extracellular turns including the second extracellular loop, which folds into the molecular center<ref name="Article6">PMID:18692154</ref>. | Somewhat enclosing this chromophore is a retinal binding pocket partially formed by the N-terminal domain overlaying the extracellular turns including the second extracellular loop, which folds into the molecular center<ref name="Article6">PMID:18692154</ref>. | ||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
==Function== | ==Function== | ||
===Visual Signal Transduction=== | ===Visual Signal Transduction=== | ||
| - | <applet load='1u19' size='300' color='black' frame='true' align='right' caption='Residues Involved in Activation of Rhodopsin. The generated structure is from Chain A.'/> | ||
====Photoisomerization of 11-''cis'' Retinal==== | ====Photoisomerization of 11-''cis'' Retinal==== | ||
The 11-''cis'' retinal (retinylidene) Schiff base functions as an [http://en.wikipedia.org/wiki/Inverse_agonist inverse agonist] and is prominently involved in the activation of rhodopsin. The primary step in rhodopsin photoactivation occurs in the photoisomerization of rhodopsin, as light energy absorbed from a photon is converted into chemical energy. As a photon is absorbed by the retina, the 11-''cis'' retinylidene ligand is switched into an all-''trans'' retinal configuration<ref name="Article2"/>. In this extremely efficient <200 fs process, the protein-binding pocket, initially fitted to accommodate the 11-''cis'' conformation of the chromophore, is preserved, which restrains the relaxation of the chromophore. The strained relaxation of conformational energy changes the protein state into the active form<ref name="Article2"/>. | The 11-''cis'' retinal (retinylidene) Schiff base functions as an [http://en.wikipedia.org/wiki/Inverse_agonist inverse agonist] and is prominently involved in the activation of rhodopsin. The primary step in rhodopsin photoactivation occurs in the photoisomerization of rhodopsin, as light energy absorbed from a photon is converted into chemical energy. As a photon is absorbed by the retina, the 11-''cis'' retinylidene ligand is switched into an all-''trans'' retinal configuration<ref name="Article2"/>. In this extremely efficient <200 fs process, the protein-binding pocket, initially fitted to accommodate the 11-''cis'' conformation of the chromophore, is preserved, which restrains the relaxation of the chromophore. The strained relaxation of conformational energy changes the protein state into the active form<ref name="Article2"/>. | ||
| Line 57: | Line 46: | ||
The excited rhodopsin interacts with a large number of transducin molecules, found in the cytoplasmic face of the disk membrane. Transducin is a member of the heterotrimeric GTP-binding proteins family, and it binds to GDP in the dark. This interaction generates a signaling cascade where transducin molecules are activated through the trigger of GDP-GTP nucleotide exchange in the α subunit<ref name="Article6"/>. Each activated transducin dissociates into Tα-GTP and Tβγ subunits, and Tα-GTP activates [http://en.wikipedia.org/wiki/CGMP-specific_phosphodiesterase_type_5 cGMP-specific phosphodiesterase] by binding and removing its inhibitory subunit<ref name="Textbook">Nelson, D., and Cox, M. Lehninger Principles of Biochemistry. 2008. 5th edition. W. H. Freeman and Company, New York, New York, USA. pp. 462-465.</ref>. | The excited rhodopsin interacts with a large number of transducin molecules, found in the cytoplasmic face of the disk membrane. Transducin is a member of the heterotrimeric GTP-binding proteins family, and it binds to GDP in the dark. This interaction generates a signaling cascade where transducin molecules are activated through the trigger of GDP-GTP nucleotide exchange in the α subunit<ref name="Article6"/>. Each activated transducin dissociates into Tα-GTP and Tβγ subunits, and Tα-GTP activates [http://en.wikipedia.org/wiki/CGMP-specific_phosphodiesterase_type_5 cGMP-specific phosphodiesterase] by binding and removing its inhibitory subunit<ref name="Textbook">Nelson, D., and Cox, M. Lehninger Principles of Biochemistry. 2008. 5th edition. W. H. Freeman and Company, New York, New York, USA. pp. 462-465.</ref>. | ||
The cGMP phosphodiesterase is an integral protein of the retina with its active site on the cytoplasmic side of the disk. Its inhibitory subunit tightly binds to it in the dark and suppresses its activity. The now activated phosphodiesterase degrades many molecules of cGMP, efficiently decreasing the concentration of cGMP<ref name="Textbook"/>. This results in the closing of the cGMP-gated cation channels in the plasma membrane of the outer segment. The cell hyperpolarizes due to the decrease in the influx of sodium and calcium ions, which results in the decrease of the release of glutamate into the synaptic cleft. This electric signal of this hyperpolarization is sent to the brain through ranks of interconnecting neurons and then through the optic nerve<ref name="Article6"/>. | The cGMP phosphodiesterase is an integral protein of the retina with its active site on the cytoplasmic side of the disk. Its inhibitory subunit tightly binds to it in the dark and suppresses its activity. The now activated phosphodiesterase degrades many molecules of cGMP, efficiently decreasing the concentration of cGMP<ref name="Textbook"/>. This results in the closing of the cGMP-gated cation channels in the plasma membrane of the outer segment. The cell hyperpolarizes due to the decrease in the influx of sodium and calcium ions, which results in the decrease of the release of glutamate into the synaptic cleft. This electric signal of this hyperpolarization is sent to the brain through ranks of interconnecting neurons and then through the optic nerve<ref name="Article6"/>. | ||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
===Visual Signal Termination=== | ===Visual Signal Termination=== | ||
| - | <applet load='1u19' size='300' color='black' frame='true' align='right' caption='Phosphorylation of Rhodospin. The generated structure is from Chain A.'/> | ||
====Recovery of the Pre-stimulus State==== | ====Recovery of the Pre-stimulus State==== | ||
In the event of a decrease in light intensity, GTP is hydrolyzed and the α-subunit of transducin reassociates with the βγ subunits, releasing the inhibitory subunit of phosphodiesterase. This subunit reassociates with phosphodiesterase and inhibits its activity<ref name="Textbook"/>. | In the event of a decrease in light intensity, GTP is hydrolyzed and the α-subunit of transducin reassociates with the βγ subunits, releasing the inhibitory subunit of phosphodiesterase. This subunit reassociates with phosphodiesterase and inhibits its activity<ref name="Textbook"/>. | ||
| Line 80: | Line 59: | ||
==Opsin== | ==Opsin== | ||
| - | <applet load='3cap' size='300' color='black' frame='true' align='right' caption='Structure of Opsin†. The generated structure is from Chain A.'/> | ||
===Topology Overview=== | ===Topology Overview=== | ||
The overall dimeric structure of opsin is similar to rhodopsin, with seven transmembrane helices linked by three extracellular loops and three cytoplasmic loops and a cytoplasmic Helix 8. The small differences between the topology of the two proteins include a short helical turn in the cytoplasmic loop 1 in opsin, 1.5-2.5 helical turns longer in Helix 5 for opsin in comparison to rhodopsin, and a large outward tilt of Helix 6 of opsin<ref name="ArticleOpsin2">PMID:18563085</ref>. Also, in contrast to rhodopsin, opsin has two openings of the retinal-binding pocket; one of the openings is between Helix 1 and Helix 7, and the other opening is between the extracellular ends of Helix 5 and 6. This opening is formed by the residues <scene name='Sandbox_173/Opsin_retinal_opening/1'>Isoleucine 205 and Phenylalanine 208 in Helix 5, and by the residues Phenylalanine 273 and Phenylalanine 276 in Helix 6</scene><ref name="ArticleOpsin2"/>. The two openings suggest different sites of retinal entrance and exit in retinal channeling<ref name="ArticleOpsin2"/>. | The overall dimeric structure of opsin is similar to rhodopsin, with seven transmembrane helices linked by three extracellular loops and three cytoplasmic loops and a cytoplasmic Helix 8. The small differences between the topology of the two proteins include a short helical turn in the cytoplasmic loop 1 in opsin, 1.5-2.5 helical turns longer in Helix 5 for opsin in comparison to rhodopsin, and a large outward tilt of Helix 6 of opsin<ref name="ArticleOpsin2">PMID:18563085</ref>. Also, in contrast to rhodopsin, opsin has two openings of the retinal-binding pocket; one of the openings is between Helix 1 and Helix 7, and the other opening is between the extracellular ends of Helix 5 and 6. This opening is formed by the residues <scene name='Sandbox_173/Opsin_retinal_opening/1'>Isoleucine 205 and Phenylalanine 208 in Helix 5, and by the residues Phenylalanine 273 and Phenylalanine 276 in Helix 6</scene><ref name="ArticleOpsin2"/>. The two openings suggest different sites of retinal entrance and exit in retinal channeling<ref name="ArticleOpsin2"/>. | ||
| Line 93: | Line 71: | ||
† PDB structure used in this section: [[3cap]] | † PDB structure used in this section: [[3cap]] | ||
| + | </StructureSection> | ||
==References== | ==References== | ||
Revision as of 09:17, 23 August 2011
| |||||||||||
References
- ↑ Hornak V, Ahuja S, Eilers M, Goncalves JA, Sheves M, Reeves PJ, Smith SO. Light activation of rhodopsin: insights from molecular dynamics simulations guided by solid-state NMR distance restraints. J Mol Biol. 2010 Feb 26;396(3):510-27. Epub 2009 Dec 11. PMID:20004206 doi:10.1016/j.jmb.2009.12.003
- ↑ 2.0 2.1 2.2 2.3 2.4 2.5 Sakmar TP. Structure of rhodopsin and the superfamily of seven-helical receptors: the same and not the same. Curr Opin Cell Biol. 2002 Apr;14(2):189-95. PMID:11891118
- ↑ 3.0 3.1 3.2 3.3 Kristiansen K. Molecular mechanisms of ligand binding, signaling, and regulation within the superfamily of G-protein-coupled receptors: molecular modeling and mutagenesis approaches to receptor structure and function. Pharmacol Ther. 2004 Jul;103(1):21-80. PMID:15251227 doi:10.1016/j.pharmthera.2004.05.002
- ↑ 4.0 4.1 4.2 Meng EC, Bourne HR. Receptor activation: what does the rhodopsin structure tell us? Trends Pharmacol Sci. 2001 Nov;22(11):587-93. PMID:11698103
- ↑ 5.0 5.1 Shieh T, Han M, Sakmar TP, Smith SO. The steric trigger in rhodopsin activation. J Mol Biol. 1997 Jun 13;269(3):373-84. PMID:9199406 doi:10.1006/jmbi.1997.1035
- ↑ 6.0 6.1 6.2 6.3 6.4 6.5 6.6 6.7 6.8 Okada T, Ernst OP, Palczewski K, Hofmann KP. Activation of rhodopsin: new insights from structural and biochemical studies. Trends Biochem Sci. 2001 May;26(5):318-24. PMID:11343925
- ↑ 7.0 7.1 Okada T, Sugihara M, Bondar AN, Elstner M, Entel P, Buss V. The retinal conformation and its environment in rhodopsin in light of a new 2.2 A crystal structure. J Mol Biol. 2004 Sep 10;342(2):571-83. PMID:15327956 doi:10.1016/j.jmb.2004.07.044
- ↑ 8.0 8.1 Janz JM, Farrens DL. Assessing structural elements that influence Schiff base stability: mutants E113Q and D190N destabilize rhodopsin through different mechanisms. Vision Res. 2003 Dec;43(28):2991-3002. PMID:14611935
- ↑ 9.0 9.1 9.2 Kisselev OG. Focus on molecules: rhodopsin. Exp Eye Res. 2005 Oct;81(4):366-7. PMID:16051215 doi:10.1016/j.exer.2005.06.018
- ↑ 10.0 10.1 10.2 Verhoeven MA, Bovee-Geurts PH, de Groot HJ, Lugtenburg J, DeGrip WJ. Methyl substituents at the 11 or 12 position of retinal profoundly and differentially affect photochemistry and signalling activity of rhodopsin. J Mol Biol. 2006 Oct 13;363(1):98-113. Epub 2006 Jul 28. PMID:16962138 doi:10.1016/j.jmb.2006.07.039
- ↑ 11.0 11.1 11.2 11.3 Morris MB, Dastmalchi S, Church WB. Rhodopsin: structure, signal transduction and oligomerisation. Int J Biochem Cell Biol. 2009 Apr;41(4):721-4. Epub 2008 Aug 3. PMID:18692154 doi:10.1016/j.biocel.2008.04.025
- ↑ 12.0 12.1 12.2 12.3 12.4 Nelson, D., and Cox, M. Lehninger Principles of Biochemistry. 2008. 5th edition. W. H. Freeman and Company, New York, New York, USA. pp. 462-465.
- ↑ Hurley JB, Spencer M, Niemi GA. Rhodopsin phosphorylation and its role in photoreceptor function. Vision Res. 1998 May;38(10):1341-52. PMID:9667002
- ↑ 14.0 14.1 14.2 Park JH, Scheerer P, Hofmann KP, Choe HW, Ernst OP. Crystal structure of the ligand-free G-protein-coupled receptor opsin. Nature. 2008 Jul 10;454(7201):183-7. Epub 2008 Jun 18. PMID:18563085 doi:10.1038/nature07063
- ↑ 15.0 15.1 Surya A, Knox BE. Enhancement of opsin activity by all-trans-retinal. Exp Eye Res. 1998 May;66(5):599-603. PMID:9628807 doi:10.1006/exer.1997.0453
Proteopedia Page Contributors and Editors (what is this?)
Michal Harel, Alexander Berchansky, Wayne Decatur, Jaime Prilusky, Joel L. Sussman, Cinting Lim