Luciola cruciata luciferase
From Proteopedia
(→Related Links) |
(→Related Links) |
||
| Line 16: | Line 16: | ||
== Related Links == | == Related Links == | ||
| + | [http://www.proteopedia.org/wiki/index.php/Luciferase List of Proteopedia pages related to Luciferases] | ||
| + | |||
[http://www.pymol.org/ Pymol molecular viewer] | [http://www.pymol.org/ Pymol molecular viewer] | ||
Revision as of 23:41, 21 August 2010
| |||||||||
| 2d1s, resolution 1.30Å () | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Ligands: | , | ||||||||
| Non-Standard Residues: | |||||||||
| Activity: | Photinus-luciferin 4-monooxygenase (ATP-hydrolyzing), with EC number 1.13.12.7 | ||||||||
| Related: | 2d1r, 2d1t | ||||||||
| |||||||||
| |||||||||
| Resources: | FirstGlance, OCA, PDBsum, RCSB, TOPSAN | ||||||||
| Coordinates: | save as pdb, mmCIF, xml | ||||||||
Contents |
Introduction
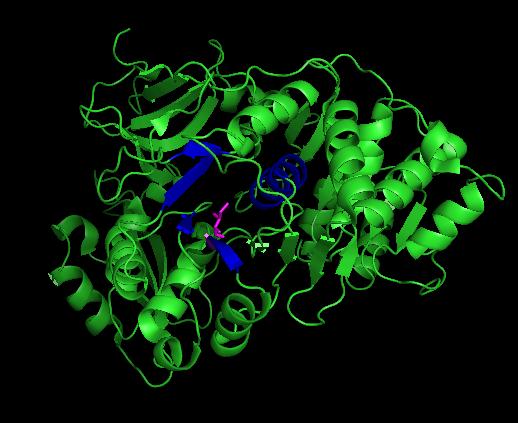
Bioluminescence is utilized by several nocturnal japanese firely species during mate selection, with males and females illuminating equally. Several common signals appear to be used to communicate everything from "male awaiting a mate" to "female here". [1] While the reaction is quite similiar to that of other bioluminescent luciferases, firefly luciferase has a unique structure in both the protein and luciferin required to produce the bioluminescence. In research, the firefly luciferase from Luciola cruciata is one of many commonly utilized for such purposes as such as sensing cellular ATP levels or visualizing the effects of a promoter sequence, among several others.
Structure
Generally, firefly luciferases have some similarities with Acyl-CoA ligases and some peptide synthetases despite having different cellular effects. In fixing the structure of L. cruciata luciferase, the analog of a potent aminoacyl-tRNA synthetases (DLSA) was successfuly utilized to represent a stable oxyluciferin intermediate.[2]. The DLSA occupied the active site of the luciferase, which is composed of an α-helix (residues 248-260) and four short β-sheets (residues 286-289, 313-316, 339-342 and 351-353. Ile288 has been implicated as an important residue in determining the hydrophobicity of the active site environment, and through orientation of the product oxyluciferin, the bioluminescent colour. [2].
Luciferase Control
As the structure of luciferases differ between species, so does the method of control over the bioluminescent reaction. In L. polyedrum, a marine dinoflagellate responsible for some red tides, a pH-dependant mechanism at the protein level appears to be responsible for control of bioluminescence. With fireflies however, the reaction is under at least some form of nervous control, with the insect controlling flashes through the use of nitric oxide [3].
Related Links
List of Proteopedia pages related to Luciferases
Protein Data Bank file on 2D1S
NCBI protein entry on Photinus pyralis luciferase, the american firefly
Proteopedia entry on dinoflagellate luciferase
References
- ↑ Suzuki H, Sato Y, Fujiyama S, Ohba N. Biochemical systematics of Japanese fireflies of the subfamily Luciolinae and their flash communication systems. Biochem Genet. 1996 Jun;34(5-6):191-200. PMID:8813052
- ↑ 2.0 2.1 Nakatsu T, Ichiyama S, Hiratake J, Saldanha A, Kobashi N, Sakata K, Kato H. Structural basis for the spectral difference in luciferase bioluminescence. Nature. 2006 Mar 16;440(7082):372-6. PMID:16541080 doi:10.1038/nature04542
- ↑ Trimmer BA, Aprille JR, Dudzinski DM, Lagace CJ, Lewis SM, Michel T, Qazi S, Zayas RM. Nitric oxide and the control of firefly flashing. Science. 2001 Jun 29;292(5526):2486-8. PMID:11431567 doi:10.1126/science.1059833
Proteopedia Page Contributors and Editors (what is this?)
Loïc Gazquez, Marion Bonazzi, Wayne Decatur, Alexander Berchansky, James Jones, David Canner, Michal Harel, Jaime Prilusky