2qjm
From Proteopedia
(New page: 200px<br /><applet load="2qjm" size="350" color="white" frame="true" align="right" spinBox="true" caption="2qjm, resolution 2.20Å" /> '''Crystal structure of...) |
|||
| Line 4: | Line 4: | ||
==Overview== | ==Overview== | ||
| - | The d-mannonate dehydratase (ManD) function was assigned to a group of | + | The d-mannonate dehydratase (ManD) function was assigned to a group of orthologous proteins in the mechanistically diverse enolase superfamily by screening a library of acid sugars. Structures of the wild type ManD from Novosphingobium aromaticivorans were determined at pH 7.5 in the presence of Mg2+ and also in the presence of Mg2+ and the 2-keto-3-keto-d-gluconate dehydration product; the structure of the catalytically active K271E mutant was determined at pH 5.5 in the presence of the d-mannonate substrate. As previously observed in the structures of other members of the enolase superfamily, ManD contains two domains, an N-terminal alpha+beta capping domain and a (beta/alpha)7beta-barrel domain. The barrel domain contains the ligands for the essential Mg2+, Asp 210, Glu 236, and Glu 262, at the ends of the third, fourth, and fifth beta-strands of the barrel domain, respectively. However, the barrel domain lacks both the Lys acid/base catalyst at the end of the second beta-strand and the His-Asp dyad acid/base catalyst at the ends of the seventh and sixth beta-strands, respectively, that are found in many members of the superfamily. Instead, a hydrogen-bonded dyad of Tyr 159 in a loop following the second beta-strand and Arg 147 at the end of the second beta-strand are positioned to initiate the reaction by abstraction of the 2-proton. Both Tyr 159 and His 212, at the end of the third beta-strand, are positioned to facilitate both syn-dehydration and ketonization of the resulting enol intermediate to yield the 2-keto-3-keto-d-gluconate product with the observed retention of configuration. The identities and locations of these acid/base catalysts as well as of cationic amino acid residues that stabilize the enolate anion intermediate define a new structural strategy for catalysis (subgroup) in the mechanistically diverse enolase superfamily. With these differences, we provide additional evidence that the ligands for the essential Mg2+ are the only conserved residues in the enolase superfamily, establishing the primary functional importance of the Mg2+-assisted strategy for stabilizing the enolate anion intermediate. |
==About this Structure== | ==About this Structure== | ||
| Line 13: | Line 13: | ||
[[Category: Novosphingobium aromaticivorans]] | [[Category: Novosphingobium aromaticivorans]] | ||
[[Category: Single protein]] | [[Category: Single protein]] | ||
| - | [[Category: Almo, S | + | [[Category: Almo, S C.]] |
| - | [[Category: Fedorov, A | + | [[Category: Fedorov, A A.]] |
| - | [[Category: Fedorov, E | + | [[Category: Fedorov, E V.]] |
| - | [[Category: Gerlt, J | + | [[Category: Gerlt, J A.]] |
| - | [[Category: Rakus, J | + | [[Category: Rakus, J F.]] |
| - | [[Category: Vick, J | + | [[Category: Vick, J E.]] |
[[Category: CS2]] | [[Category: CS2]] | ||
[[Category: MG]] | [[Category: MG]] | ||
| Line 25: | Line 25: | ||
[[Category: lyase]] | [[Category: lyase]] | ||
| - | ''Page seeded by [http://oca.weizmann.ac.il/oca OCA ] on | + | ''Page seeded by [http://oca.weizmann.ac.il/oca OCA ] on Thu Feb 21 18:39:50 2008'' |
Revision as of 16:39, 21 February 2008
|
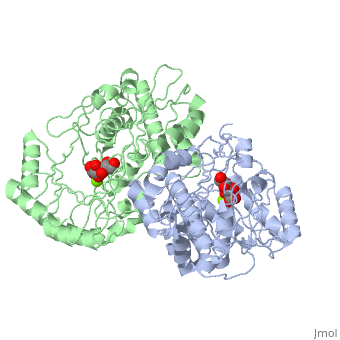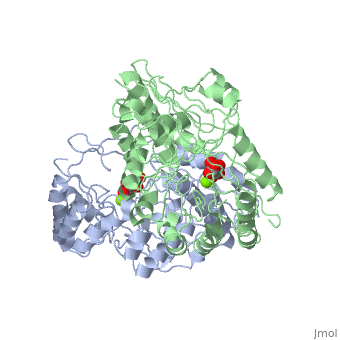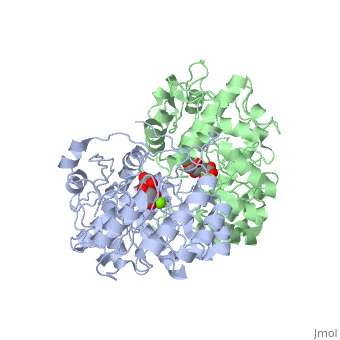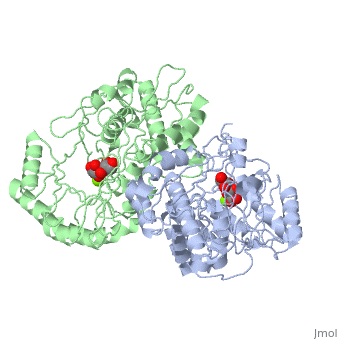
Crystal structure of the K271E mutant of Mannonate dehydratase from Novosphingobium aromaticivorans complexed with Mg and D-mannonate
Overview
The d-mannonate dehydratase (ManD) function was assigned to a group of orthologous proteins in the mechanistically diverse enolase superfamily by screening a library of acid sugars. Structures of the wild type ManD from Novosphingobium aromaticivorans were determined at pH 7.5 in the presence of Mg2+ and also in the presence of Mg2+ and the 2-keto-3-keto-d-gluconate dehydration product; the structure of the catalytically active K271E mutant was determined at pH 5.5 in the presence of the d-mannonate substrate. As previously observed in the structures of other members of the enolase superfamily, ManD contains two domains, an N-terminal alpha+beta capping domain and a (beta/alpha)7beta-barrel domain. The barrel domain contains the ligands for the essential Mg2+, Asp 210, Glu 236, and Glu 262, at the ends of the third, fourth, and fifth beta-strands of the barrel domain, respectively. However, the barrel domain lacks both the Lys acid/base catalyst at the end of the second beta-strand and the His-Asp dyad acid/base catalyst at the ends of the seventh and sixth beta-strands, respectively, that are found in many members of the superfamily. Instead, a hydrogen-bonded dyad of Tyr 159 in a loop following the second beta-strand and Arg 147 at the end of the second beta-strand are positioned to initiate the reaction by abstraction of the 2-proton. Both Tyr 159 and His 212, at the end of the third beta-strand, are positioned to facilitate both syn-dehydration and ketonization of the resulting enol intermediate to yield the 2-keto-3-keto-d-gluconate product with the observed retention of configuration. The identities and locations of these acid/base catalysts as well as of cationic amino acid residues that stabilize the enolate anion intermediate define a new structural strategy for catalysis (subgroup) in the mechanistically diverse enolase superfamily. With these differences, we provide additional evidence that the ligands for the essential Mg2+ are the only conserved residues in the enolase superfamily, establishing the primary functional importance of the Mg2+-assisted strategy for stabilizing the enolate anion intermediate.
About this Structure
2QJM is a Single protein structure of sequence from Novosphingobium aromaticivorans with and as ligands. Full crystallographic information is available from OCA.
Reference
Evolution of enzymatic activities in the enolase superfamily: D-Mannonate dehydratase from Novosphingobium aromaticivorans., Rakus JF, Fedorov AA, Fedorov EV, Glasner ME, Vick JE, Babbitt PC, Almo SC, Gerlt JA, Biochemistry. 2007 Nov 13;46(45):12896-908. Epub 2007 Oct 18. PMID:17944491
Page seeded by OCA on Thu Feb 21 18:39:50 2008