|
|
| Line 1: |
Line 1: |
| | =[Image:Opening 1igt.png|450px|left|thumb| Intact Immunoglobulin, [[1igt]]]] | | =[Image:Opening 1igt.png|450px|left|thumb| Intact Immunoglobulin, [[1igt]]]] |
| - | {{STRUCTURE_1hzh| right| PDB=1hzh | SCENE=Antibody/1hzh_starting_scene/3 |CAPTION= Crystal Structure of the Intact Human IGG B12: A Template for a Potential HIV Vaccine, [[1hzh]] }} | + | {{STRUCTURE_1hzh| right| PDB=1hzh | SCENE=Antibody/1hzh_starting_scene/3 |CAPTION= Structura IGG B12 uman: o monstra pentru un potential vaccin HIV, [[1hzh]] }} |
| | '''Antibodies''', also known as Immunoglobulins (Ig) are gamma globulin proteins, primarily found in the blood of vertebrates. These [[glycoproteins]] serve as a critical component of the immune system when the host fails to activate alternative compliment pathways or phagocytic cells in response to invading microorganisms or other [http://en.wikipedia.org/wiki/Antigen antigens]. The incredible specificity with which immunoglobulins bind to an antigen is based upon structural complementarity between the antigen and antibody <scene name='Antibody/1hzh_heavy_chains/1'>heavy </scene>and <scene name='Antibody/1hzh_light_chains/1'>light chains </scene>. It is this specificity that has made <scene name='Antibody/1hzh_starting_scene/3'>antibodies</scene> a critical component in laboratory and medical research. | | '''Antibodies''', also known as Immunoglobulins (Ig) are gamma globulin proteins, primarily found in the blood of vertebrates. These [[glycoproteins]] serve as a critical component of the immune system when the host fails to activate alternative compliment pathways or phagocytic cells in response to invading microorganisms or other [http://en.wikipedia.org/wiki/Antigen antigens]. The incredible specificity with which immunoglobulins bind to an antigen is based upon structural complementarity between the antigen and antibody <scene name='Antibody/1hzh_heavy_chains/1'>heavy </scene>and <scene name='Antibody/1hzh_light_chains/1'>light chains </scene>. It is this specificity that has made <scene name='Antibody/1hzh_starting_scene/3'>antibodies</scene> a critical component in laboratory and medical research. |
| | | | |
| Line 7: |
Line 7: |
| | [[Image:230px-B cell activation2.png|270px|left|thumb| Production of Antibodies by Plasma Cells]] | | [[Image:230px-B cell activation2.png|270px|left|thumb| Production of Antibodies by Plasma Cells]] |
| | | | |
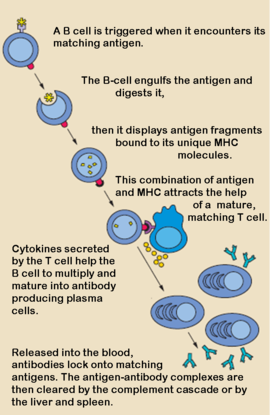
| - | ==Cellular Basis of Antibody Production== | |
| - | When a foreign antigen binds to a B-lymphocyte ([http://en.wikipedia.org/wiki/B_cell B-cell]), it activates the B-cell, and upon stimulation by [http://en.wikipedia.org/wiki/Helper_t_cell helper T-cells], undergoes clonal proliferation and B-cell maturation into antibody forming [http://en.wikipedia.org/wiki/Plasma_cells plasma cells]. Each plasma cell is programmed to make an antibody of a single specificity, which it releases into the blood. <ref name="Roit"> Roit, I. M. Roit's Essential Immunology. Oxford: Blackwell Science Ltd., 1997.</ref> Once in the blood, antibodies aid [http://en.wikipedia.org/wiki/Humoral_immune_system the humoral immune system] in three predominant ways: They coat foreign pathogens preventing them from entering healthy cells or disrupting antigen function; they coat pathogens, stimulating their removal via [http://en.wikipedia.org/wiki/Opsonization opsonization] by [http://en.wikipedia.org/wiki/Phagocytes phagocytes]; and they trigger destruction of pathogens by stimulating the [http://en.wikipedia.org/wiki/Complement_system complement pathway] or by [http://en.wikipedia.org/wiki/Antibody-dependent_cellular_cytotoxicity Antibody Dependent Cell-mediated Cytotoxicity], among other immune responses. <ref>PMID:8476565</ref> <ref>PMID:16234578</ref> All of these functions rely heavily on accurate antigen binding and communication with other immune effector cells. The amazing specificity antibodies operate with is made possible by the physical structure of the antibody, which appears simplistic, but contains several levels of additional complexity. | |
| | | | |
| | ==Structure of the Immunoglobulin== | | ==Structure of the Immunoglobulin== |
| | {{STRUCTURE_1igt| right| |size=400| PDB=1igt | Size=400px| SCENE=Antibody/1igt_starting_scene/3 |CAPTION= Refined Structure of an Intact IgG2a Monoclonal Antibody, [[1igt]] }} | | {{STRUCTURE_1igt| right| |size=400| PDB=1igt | Size=400px| SCENE=Antibody/1igt_starting_scene/3 |CAPTION= Refined Structure of an Intact IgG2a Monoclonal Antibody, [[1igt]] }} |
| - | The basic functional unit of an antibody is an immunoglobulin monomer, but antibodies secreted from plasma cells are typically dimeric with occasional higher order structures. Typical secreted antibodies have a basic four-peptide structure of two identical <scene name='Antibody/1igt_heavy_chains/1'>heavy chains </scene>and two identical <scene name='Antibody/1igt_light_chains/1'>light chains</scene> joined together by interchain <scene name='Antibody/1igt_disulfide_bonds/2'>disulfide bonds</scene>, forming a “Y” shaped molecule. The disulfide bonds are positioned within a flexible region called the <scene name='Antibody/1igt_hinge_region/1'>hinge region</scene>, which seperates the lobes of the antibody from one another and provides ample flexibility to bind antigens effectively. <ref name="Roit" /> Each domain (2 heavy and 2 light) contain between 70-110 amino acids and are classified into different categories according to size and function. <ref>PMID:10545762</ref> Both domains, heavy and light, contain variable and constant regions that are crucial to antibody function. <ref>PMID:107164</ref> | + | The basic functional unit of an antibody is an immunoglobulin monomer, but antibodies secreted from plasma cells are typically dimeric with occasional higher order structures. Typical secreted antibodies have a basic four-peptide structure of two identical <scene name='Antibody/1igt_heavy_chains/1'>heavy chains </scene>and two identical <scene name='Antibody/1igt_light_chains/1'>light chains</scene> joined together by interchain <scene name='Antibody/1igt_disulfide_bonds/2'>disulfide bonds</scene>, forming a “Y” shaped molecule. The disulfide bonds are positioned within a flexible region called the <scene name='Antibody/1igt_hinge_region/1'>hinge region</scene>, which seperates the lobes of the antibody from one another and provides ample flexibility to bind antigens effectively. <ref name="Roit" /> Each domain (2 heavy and 2 light) contain between 70-110 amino acids and are classified into different categories according to size and function. <ref>PMID:10545762</ref> Both domains, heavy and light, contain variable and constant regions that are crucial to antibody function. <ref>PMID:107164</ref> |
| - | | + | |
| - | ===Heavy Chain===
| + | |
| - | There are five types of immunoglobulin heavy chains, in mammals, α, δ, ε, γ, and μ, and give rise to the five unique classes or isotypes of antibodies, IgA, IgD, IgE, IgG, and IgM, which differ in size and composition. Each <scene name='Antibody/1igt_heavy_chains_ribbon/1'>heavy chain </scene>has a <scene name='Antibody/1igt_startconstant_region/1'>constant region </scene> and <scene name='Antibody/1igt_start_heavy_variable/1'>variable region</scene>. The constant region is identical in all antibodies of the same isotype, but differ in antibodies of different isotypes; i.e. all IgA have the same sequence in their heavy chain constant region, but these constant regions differ between IgA and IgD, etc. <ref>PMID:15040582</ref> The α, δ, and γ heavy chains have a constant region composed of <scene name='Antibody/1igt_heavy_3_chains/1'>three tandem immunoglobulin domains</scene> while heavy chains ε and μ contain four. The variable region of the heavy chain in antibodies is different for all antibodies created by different B-cells. <ref>PMID:107164</ref>
| + | |
| - | | + | |
| - | [[Image:New Antibody2.JPG|450px|left|thumb| Typical Structure of an Antibody]]
| + | |
| - | | + | |
| - | ===Light Chain===
| + | |
| - | Every antibody contains two <scene name='Antibody/1igt_light_chains_ribbon/1'>light chains </scene>that are identical to each other. There are two types of immunoglobulin light chains in mammals, labeled lambda and kappa, with only one represented in each antibody. Each light chain has one <scene name='Antibody/1igt_light_chains_constnat_reg/1'>constant domain </scene>followed by one <scene name='Antibody/1igt_light_chain_variable/1'>variable domain</scene>, with a total length of about 215 amino acids. <ref name="Roit" />
| + | |
| - | | + | |
| - | === The Regions: Fab, Fv, CDR, and Fc.===
| + | |
| - | The immunoglobulin can be broken down into regions, each serving a different purpose:
| + | |
| - | | + | |
| - | ====Variable Regions====
| + | |
| - | The <scene name='Antibody/1igt_fab_region/1'>Fab region </scene>(Fragment, Antigen Binding region) is composed of one constant and one variable domain from each heavy and light chain of the antibody. It is the part of the antibody that gives it its famous “Y” shape.<ref>PMID:9048542</ref> Held within the Fab region is the variable domain, also known as the Fv region.<ref>PMID:4569769</ref> Within the Fv region lie <scene name='Antibody/1igt_start_variable_loops/1'>“hypervariable regions,”</scene> positioned at one end of the variable domain where they form parts of the Beta-turn loops and are clustered close to each other in space. The clustering of the hypervariable loops at the tips of the variable regions where the antigen-binding site is located makes them perfect candidates for antigen recognition. <ref name="Roit" />The sequence heterogeneity of the three heavy and three light chain hypervariable loops creates significant antigen specificity diversity through variations in the binding surface nature and shape. Each hypervariable region can be viewed as an independent structure contributing to the complementarity of the biding site and antigen and is often referred to as a complementarity determining region (CDR). <ref>PMID:107164</ref>
| + | |
| - | | + | |
| - | ====Constant Regions====
| + | |
| - | The remaining part of the antibody, namely the <scene name='Antibody/1igt_fc/2'>Fc region</scene>, does not play a role in binding the antigen, but rather is responsible for modulating the immune systems response to the formation of an antibody-antigen complex. The Fragment Crystallizable (Fc) region is composed of two heavy chain constant regions that are isotype specific. <ref>PMID:15040582</ref> Antibodies are glycoproteins because of <scene name='Antibody/1igt_glycosylation/1'>glycosylation </scene>at conserved positions in their Fc regions. This glycosylation is a critical component determing the rate of antibody clearance form the body.<ref>PMID:9032990</ref> Once an antibody binds to an antigen, the Fc region binds to Fc receptors, among other proteins, to mediate a host of different physiological responses ranging from oposonization, to degranulation of mast cells, to the release of cytokines and cytotoxic molecules, etc. resulting in the destruction of the pathogen. <ref>PMID:9052877</ref> Depending on the class of antibody, as dictated by the identity of the Fc region, the antibody half-life and distribution throughout the body varies. Further, since Fc receptors are antibody isotype specific, the type of immune response is dependent on the type of Fc region on the immunoglobulin, allowing for different immune responses to the same pathogen if necessary.<ref>PMID:11244038</ref> See table for brief characterization of Immunoglobulin isotypes:
| + | |
| - | | + | |
| - | {| class="wikitable" border="1" width="70%" style="text-align:center"
| + | |
| - | |-
| + | |
| - | ! colspan="2" align="center"| Immunoglobulin Classes and Function
| + | |
| - | |-
| + | |
| - | ! Class
| + | |
| - | ! Function and Oligomeric State<ref name="Roit" />
| + | |
| - | |-
| + | |
| - | ! IgG
| + | |
| - | | Dimeric - The most abundant Ig in the extravascular fluids. Neutralizes toxins and combats microorganisms by activating the compliment system and facilitating the binding of phagocytic cells.
| + | |
| - | |-
| + | |
| - | ! [[IgA]]
| + | |
| - | | Dimeric - Is the major Ig in seromucous secretions, where it serves to defend the external body surfaces.
| + | |
| - | |-
| + | |
| - | ! IgM
| + | |
| - | | Pentameric – It is an intravascular antibody and is produced very early in the immune response. Due to it high oligomeric state, it is extremely effective as a bacterial agglutinator and mediator of complement-dependent cytolysis, making it a powerful first-line defense against bacterial pathogens.
| + | |
| - | |-
| + | |
| - | ! IgD
| + | |
| - | | Dimeric - It is present on the lymphocyte and functions together with IgM as the antigen receptor on naïve B-cells.
| + | |
| - | |-
| + | |
| - | ! IgE
| + | |
| - | | It binds to mast cells and upon contact with antigen, leads to local recruitment of antimicrobial agents via degranulation of the mast cell and release of inflammatory mediators. IgE is important for certain kinds of parasitic infections and is responsible for the symptoms of [http://en.wikipedia.org/wiki/Atopy atopic allergies] like eczema and asthma.
| + | |
| - | |}
| + | |
| - | | + | |
| - | A model of the IgG molecule is present in the figure which indicates the spatial disposition and interaction of the domains in IgG. As Dr. Ivan Roitt writes in Essential Immunolgy, “To enable the Fab arms to have the freedom to move and twist so that they can align their hypervariable regions with the antigenic sites on large immobile carriers, and to permit the Fc structures to adjust spatially in order to trigger their effector functions, it is desirable for IgG to have a high degree of flexibility. And it has just that. Structural analysis shows that the Fab can ‘elbow-bend’ at its V-C junction and twist about the hinge, which itself can more properly be described as a loose thether, allowing the Fab and the Fc to drift relative to each other with remarkable suppleness. It could be said that movements like that make it a very sexy molecule!” <ref name="Roit" />
| + | |
| - | | + | |
| - | | + | |
| - | [[Image:VDJ recombination.png|400px|left|thumb| Image of V(D)J Recombination]]
| + | |
| - | <applet load="Object_Monomer.pdb" size="300" color="white" frame="true" spin="on" Scene ="Antibody/Rituxan_starting_scene/1" caption="Crystal structure of Rituximab Fab in complex with an epitope peptide, [[2osl]]" align="right"/>
| + | |
| - | | + | |
| - | === Antibody Diversity ===
| + | |
| - | Considering the nearly infinite number of possible antigens that can invade the body, the immune system had to develop a method for accurately targeting each one of these compounds, ranging from small molecules, to stray proteins, to viruses capable of infecting cells. The antibody was the immune systems response to this problem. It has been estimated that humans generate about 10^10 different antigens, each capable of binding a unique epitope of an antigen. Since antibodies are proteins, and proteins are controlled by the genes from which they are transcribed, a clever system of gene shuffling and manipulations developed to enable the immune system to create a huge repertoire of antibodies from a limited number of genes. <ref>PMID:8612345</ref> The variable region of each immunoglobulin chain is encoded in several pieces known as gene segments. For heavy chains, these segments are called the variable (V), diversity (D), and joining (J) segments. (Only V and J exist for light chains) 50 V segments, 25 D segments, and 6 J segments exist and are randomly arranged and rearranged in the genome in a process called [http://en.wikipedia.org/wiki/VDJ_recombination V(D)J recombination]. Each B-cell is programmed to produce antibodies of a single V(D)J recombination order.
| + | |
| - | | + | |
| - | Additional diversity is created by the proteins RAG-1 and RAG-2 which introduce the double stranded breaks between V, D, and J segments to allow recombination. At this stage, nucleotides can either be deleted or inserted between adjoining segments before being ligated together. <ref name="Roit" /> This dramatically increases antibody diversity. Further diversity is created during B-cell proliferation when the variable chains undergo a high rate of point mutations in a process called [http://en.wikipedia.org/wiki/Somatic_hypermutation somatic hypermutation], creating daughter cells of the original B-cell that are slightly different. The antibodies which bind the antigen with the highest affinity are selected for in a process called [http://en.wikipedia.org/wiki/Affinity_maturation affinity maturation]. <ref>PMID:11869898</ref><ref>PMID:17337763</ref> Isotype switching is also possible after activation of the B-cell by a mechanism called “class switch recombination” allowing different immunological responses to the same antigen bound by the same variable regions.<ref>PMID:12884279</ref> Through this clever system, tens of billions of different glycoprotein antibodies can be created from less than 100 genes, allowing antibodies to bind <scene name='Antibody/Rituxan_binding_site/1'>structurally complimentary antigens</scene> with exquisite precision. The discovery of antobdy diversity generation won Susumu Tonegawa the [http://nobelprize.org/nobel_prizes/medicine/laureates/1987/press.html Nobel Prize in Medicine in 1987].
| + | |
| - | | + | |
| - | [[Image:FluorescentCells.jpg|300px|right|thumb| Direct Immuno fluorescence Antibody labeling]]
| + | |
| - | ==Antibody Applications==
| + | |
| - | Detection of particular antibodies is very common in medical diagnostic testing. Numerous biochemical assays exist to detect whether antibodies for specific antigens are present in the blood or other bodily fluids such as antibodies against [http://en.wikipedia.org/wiki/Lyme_disease Lyme disease] or [http://en.wikipedia.org/wiki/HIV HIV], etc. Another common medical test involving antibodies is blood type detection in which an individual’s blood is screened against anti-A and anti-B antibodies to determine the identity of that individual’s [http://en.wikipedia.org/wiki/Blood_type blood antigen type]. <ref>PMID:13477267</ref>
| + | |
| - | | + | |
| - | Antibodies are also extremely powerful tools in the laboratory setting where they are commonly used in [http://en.wikipedia.org/wiki/Western_blot Western Blot] to detect specific proteins in a sample <ref>PMID:6266278</ref>; [http://en.wikipedia.org/wiki/Flow_cytometry flow cytometry], to differentiate cell types by their protein expression profiles; [http://en.wikipedia.org/wiki/Immunoprecipitation immunoprecipitation], to separate proteins from other compounds in a [http://en.wikipedia.org/wiki/Lysate lysate] and for cellular labeling. Numerous other examples exist. <ref>PMID:15353569</ref>
| + | |
| - | | + | |
| - | The last two decades have seen a dramatic increase in antibody based technologies both for the lab and medicine thanks to the invention of the monoclonal antiboy, a discovery that won Niels K. Jerne, Georges J.F. Köhler, César Milstein the [http://nobelprize.org/nobel_prizes/medicine/laureates/1984/press.html Nobel Prize in Medicine in 1984]. See: [[Monoclonal Antibody]] for additional information.
| + | |
| - | | + | |
| - | == Additional 3D Structures of the Immunoglobulin ==
| + | |
| - | | + | |
| - | ===Full Immunoglobulin===
| + | |
| - | [[1igt]] – Anti-canine lymphoma monoclonal antibody <br />
| + | |
| - | [[1hzh]] - Crystal Structure of the Intact Human IgG B12<br />
| + | |
| - | [[1igy]] - Crystallographic structure of an intact IgG1 monoclonal antibody<br />
| + | |
| - | | + | |
| - | ===Fab Fragments===
| + | |
| - | [[3mlr]],[[3mls]],[[3mlt]],[[3mlu]],[[3mlv]],[[3mlw]],[[3mlx]],[[3mly]],[[3mlz]] - Crystal structure of anti-HIV-1 V3 Fab 2557 in complex with a NY5 V3 peptide<br />
| + | |
| - | [[3mug]] - Crystal structure of human Fab PG16, a broadly reactive and potent HIV-1 neutralizing antibody.<br />
| + | |
| - | [[3hmw]] - Crystal structure of ustekinumab FAB<br />
| + | |
| - | [[3hmx]] - Crystal structure of ustekinumab FAB/IL-12 complex<br />
| + | |
| - | [[1aqk]] - Three-dimensional structure of a human Fab with high affinity for tetanus toxoid<br />
| + | |
| - | [[1ay1]] - Anti-Taq Fab TP7<br />
| + | |
| - | [[1bbj]] - Crystal structure of a chimeric Fab' fragment of an antibody binding tumour cells<br />
| + | |
| - | [[1bm3]] - Immunoglobulin OPG2 Fab-Peptide Complex<br />
| + | |
| - | [[1jnh]],[[1jnl]] - Crystal Structure of Fab-Estradiol Complexes<br />
| + | |
| - | | + | |
| - | ===Fc Fragments===
| + | |
| - | [[1fcc]] - Crystal structure of the C2 fragment of streptococcal protein G in complex with the Fc domain of human IgG<br />
| + | |
| - | [[1f6a]] - Structure of the Fc fragment of human IgE bound to its high-affinity receptor Fc (epsilon) RI (alpha)<br />
| + | |
| - | [[1e4k]] - Crystal Structure of the Human Igg1 Fc Fragment-Fc-Gamma-Riii Complex<br />
| + | |
| - | [[1fp5]] - Structure of the human IgE-Fc C epsilon 3-C epsilon 4<br />
| + | |
| - | [[1l6x]] - Fc Fragment of Rituximab Bound to a minimized version of the B-Domain from Protein A<br />
| + | |
| - | | + | |
| - | ==References==
| + | |
| - | <references />
| + | |
| - | | + | |
| - | ==Additional Pages==
| + | |
| - | * [[IgA]]
| + | |
| - | * [[Epitopes]]
| + | |
| - | * [[Major Histocompatibility Complex Class I]]
| + | |
| - | * [[Monoclonal Antibody]]
| + | |