We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
Sandbox caroldavila
From Proteopedia
(Difference between revisions)
| Line 1: | Line 1: | ||
=[Image:Opening 1igt.png|450px|left|thumb| Intact Immunoglobulin, [[1igt]]]] | =[Image:Opening 1igt.png|450px|left|thumb| Intact Immunoglobulin, [[1igt]]]] | ||
| - | {{STRUCTURE_1hzh| right| PDB=1hzh | SCENE=Antibody/1hzh_starting_scene/3 |CAPTION= Structura IGG B12 uman: o monstra pentru un potential vaccin HIV, [[1hzh]] }} | + | {{STRUCTURE_1hzh| right| PDB=1hzh | SCENE= Antibody/1hzh_starting_scene/3 |CAPTION= Structura IGG B12 uman: o monstra pentru un potential vaccin HIV, [[1hzh]] }} |
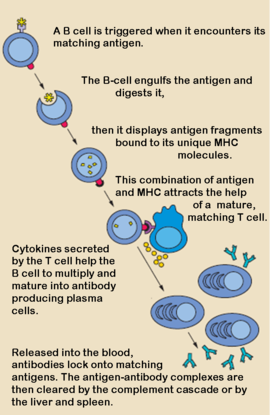
'''Antibodies''', also known as Immunoglobulins (Ig) are gamma globulin proteins, primarily found in the blood of vertebrates. These [[glycoproteins]] serve as a critical component of the immune system when the host fails to activate alternative compliment pathways or phagocytic cells in response to invading microorganisms or other [http://en.wikipedia.org/wiki/Antigen antigens]. The incredible specificity with which immunoglobulins bind to an antigen is based upon structural complementarity between the antigen and antibody <scene name='Antibody/1hzh_heavy_chains/1'>heavy </scene>and <scene name='Antibody/1hzh_light_chains/1'>light chains </scene>. It is this specificity that has made <scene name='Antibody/1hzh_starting_scene/3'>antibodies</scene> a critical component in laboratory and medical research. | '''Antibodies''', also known as Immunoglobulins (Ig) are gamma globulin proteins, primarily found in the blood of vertebrates. These [[glycoproteins]] serve as a critical component of the immune system when the host fails to activate alternative compliment pathways or phagocytic cells in response to invading microorganisms or other [http://en.wikipedia.org/wiki/Antigen antigens]. The incredible specificity with which immunoglobulins bind to an antigen is based upon structural complementarity between the antigen and antibody <scene name='Antibody/1hzh_heavy_chains/1'>heavy </scene>and <scene name='Antibody/1hzh_light_chains/1'>light chains </scene>. It is this specificity that has made <scene name='Antibody/1hzh_starting_scene/3'>antibodies</scene> a critical component in laboratory and medical research. | ||
| Line 8: | Line 8: | ||
| - | ==Structure of the Immunoglobulin== | ||
{{STRUCTURE_1igt| right| |size=400| PDB=1igt | Size=400px| SCENE=Antibody/1igt_starting_scene/3 |CAPTION= Refined Structure of an Intact IgG2a Monoclonal Antibody, [[1igt]] }} | {{STRUCTURE_1igt| right| |size=400| PDB=1igt | Size=400px| SCENE=Antibody/1igt_starting_scene/3 |CAPTION= Refined Structure of an Intact IgG2a Monoclonal Antibody, [[1igt]] }} | ||
| - | The basic functional unit of an antibody is an immunoglobulin monomer, but antibodies secreted from plasma cells are typically dimeric with occasional higher order structures. Typical secreted antibodies have a basic four-peptide structure of two identical <scene name='Antibody/1igt_heavy_chains/1'>heavy chains </scene>and two identical <scene name='Antibody/1igt_light_chains/1'>light chains</scene> joined together by interchain <scene name='Antibody/1igt_disulfide_bonds/2'>disulfide bonds</scene>, forming a “Y” shaped molecule. The disulfide bonds are positioned within a flexible region called the <scene name='Antibody/1igt_hinge_region/1'>hinge region</scene>, which seperates the lobes of the antibody from one another and provides ample flexibility to bind antigens effectively. <ref name="Roit" /> Each domain (2 heavy and 2 light) contain between 70-110 amino acids and are classified into different categories according to size and function. <ref>PMID:10545762</ref> Both domains, heavy and light, contain variable and constant regions that are crucial to antibody function. <ref>PMID:107164</ref> | ||
Revision as of 07:16, 20 October 2010
=[Image:Opening 1igt.png|450px|left|thumb| Intact Immunoglobulin, 1igt]]
| |||||||||
| Structura IGG B12 uman: o monstra pentru un potential vaccin HIV, 1hzh | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Ligands: | , , , , | ||||||||
| |||||||||
| |||||||||
| Resources: | FirstGlance, OCA, RCSB, PDBsum | ||||||||
| Coordinates: | save as pdb, mmCIF, xml | ||||||||
Antibodies, also known as Immunoglobulins (Ig) are gamma globulin proteins, primarily found in the blood of vertebrates. These glycoproteins serve as a critical component of the immune system when the host fails to activate alternative compliment pathways or phagocytic cells in response to invading microorganisms or other antigens. The incredible specificity with which immunoglobulins bind to an antigen is based upon structural complementarity between the antigen and antibody and . It is this specificity that has made a critical component in laboratory and medical research.