Phosphoinositide 3-Kinases
From Proteopedia
| Line 1: | Line 1: | ||
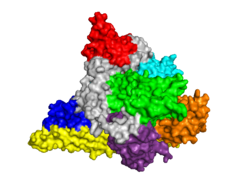
[[Image: PI3KOpener.PNG|250px|left|thumb| PI3K p110α Subunit, [[3hhm]]]] | [[Image: PI3KOpener.PNG|250px|left|thumb| PI3K p110α Subunit, [[3hhm]]]] | ||
| - | {{STRUCTURE_3hhm| right| PDB=3hhm | SCENE=Phosphoinositide_3-Kinases/Model_try/2|CAPTION= Theoretical Model of PI3K Gamma Catalytic Subunit w/ Adaptor Subunit Components, Compliments of M. Zvelebil, M.D. Waterfield(LICR, London) & Roger Williams (MRC, Cambridge)}} | + | {{STRUCTURE_3hhm| right| PDB=3hhm | SCENE=Phosphoinositide_3-Kinases/Model_try/2|CAPTION= Theoretical Model of PI3K Gamma Catalytic Subunit w/ Adaptor Subunit Components, Compliments of M. Zvelebil, M.D. Waterfield (LICR, London) & Roger Williams (MRC, Cambridge)}} |
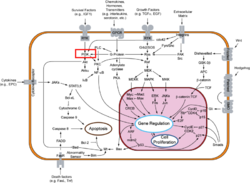
[[Phosphoinositide 3-Kinases]] (PI3K) are a family of ubiquitously distributed lipid kinases, that play a critical role in the regulation of numerous cellular processes including cellular growth and morphology, programmed cell death, cell motility and adhesion, mitogenesis and glucose uptake. <ref name="Driscoll"> PMID: 12151228</ref> PI3K generates important second messengers by catalyzing the transfer of the γ-phosphate group of ATP to the D3 position of phosphoinositides. <ref name="Wymann"> PMID: 9838078</ref> The PI3K preferred substrate is Phosphatidylinositol-4,5-bisphosphate (PIP2), which is converted into phosphatidylinositol-3,4,5-triphosphate (PIP3) upon phosphorylation at the cell membrane. The importance of PI3K is evident in knockout mice studies in which those mice with disruptions of critical PI3K components have significant deficiencies in immune and inflammatory response <ref name="Fubar"> PMID:10972292</ref> sometimes resulting in embryonic death.<ref>PMID:10196176</ref> Aberrations in PIP3 levels, either through activation of PI3ks or through inactivation of lipid phosphatase [[PTEN]], occur frequently in numerous forms of cancer, making PI3K an exciting new target to treat [[Cancer|cancer]] among other human diseases.<ref name="Miled"> PMID: 17626883</ref> | [[Phosphoinositide 3-Kinases]] (PI3K) are a family of ubiquitously distributed lipid kinases, that play a critical role in the regulation of numerous cellular processes including cellular growth and morphology, programmed cell death, cell motility and adhesion, mitogenesis and glucose uptake. <ref name="Driscoll"> PMID: 12151228</ref> PI3K generates important second messengers by catalyzing the transfer of the γ-phosphate group of ATP to the D3 position of phosphoinositides. <ref name="Wymann"> PMID: 9838078</ref> The PI3K preferred substrate is Phosphatidylinositol-4,5-bisphosphate (PIP2), which is converted into phosphatidylinositol-3,4,5-triphosphate (PIP3) upon phosphorylation at the cell membrane. The importance of PI3K is evident in knockout mice studies in which those mice with disruptions of critical PI3K components have significant deficiencies in immune and inflammatory response <ref name="Fubar"> PMID:10972292</ref> sometimes resulting in embryonic death.<ref>PMID:10196176</ref> Aberrations in PIP3 levels, either through activation of PI3ks or through inactivation of lipid phosphatase [[PTEN]], occur frequently in numerous forms of cancer, making PI3K an exciting new target to treat [[Cancer|cancer]] among other human diseases.<ref name="Miled"> PMID: 17626883</ref> | ||
__NOTOC__ | __NOTOC__ | ||
Revision as of 10:14, 10 November 2010
Template:STRUCTURE 3hhm Phosphoinositide 3-Kinases (PI3K) are a family of ubiquitously distributed lipid kinases, that play a critical role in the regulation of numerous cellular processes including cellular growth and morphology, programmed cell death, cell motility and adhesion, mitogenesis and glucose uptake. [1] PI3K generates important second messengers by catalyzing the transfer of the γ-phosphate group of ATP to the D3 position of phosphoinositides. [2] The PI3K preferred substrate is Phosphatidylinositol-4,5-bisphosphate (PIP2), which is converted into phosphatidylinositol-3,4,5-triphosphate (PIP3) upon phosphorylation at the cell membrane. The importance of PI3K is evident in knockout mice studies in which those mice with disruptions of critical PI3K components have significant deficiencies in immune and inflammatory response [3] sometimes resulting in embryonic death.[4] Aberrations in PIP3 levels, either through activation of PI3ks or through inactivation of lipid phosphatase PTEN, occur frequently in numerous forms of cancer, making PI3K an exciting new target to treat cancer among other human diseases.[5]
The Classes of PI3Ks
PI3Ks can be grouped into three distinct classes, Class I-III. Class I PI3Ks, the most well understood and thoroughly explored PI3K class, are composed of a 110kDa and a 50-100 kDa . Activation of Class I PI3Ks is controlled by extracellular signaling via receptors with intrinsic tyrosine kinase activity, G protein-linked receptors, or receptors coupled to SRC like protein tyrosine kinases. [6] Class II PI3Ks are relatively poorly understood but are 170-210 kDa and have in vitro substrate specificity toward PtdIns 4-P. Class III PI3Ks depend on Vps15p protein Ser/Thr kinases, which recruits the phosphatidylinositol kinase to late Golgi Compartments. [2]Class I Subclasses
PI3Ks are activated by extracellular agonists via the translocation of PI3Ks to the plasma membrane for easy access to lipid substrates. Depending on the adaptor proteins involved in the process, Class I PI3Ks are segregated into two subgroups. Those that associate with p85 will be directed to phosphorylated tyrosine motifs (Class IA), while PI3Kγ interacts with trimeric G proteins and the p101 protein (Class IB) [2]
Structure of PI3K
Class I PI3Ks, which are tightly regulated by tyrosine kinases, are composed of an 85kDa regulatory/adapter subunit (p85) and a 110kDa catalytic subunit (p110). [7]
Adapter Subunit
| |||||||||||
The Catalytic Subunit
| |||||||||||
Activation of Class IA PI3K
Inactive PI3Ks are rapidly activated in the presence of extracellular stimuli. Such stimuli, as discussed previously, include growth factor receptors with intrinsic protein tyrosine kinase activity, which display pYXXM motifs for p85 docking, as well as receptor substrates which are phosphorylated and interact with PI3K regulatory subunits like nSH2. PI3K can be additionally activated in cooperative processes like translocation to the plasma membrane where lipid substrates are available and by binding GTP loaded Ras to the catalytic subunit. [17]. [2]
PI3K Inhibition
| |||||||||||
The Phosphorylated Lipid Products in Downstream Signaling
Ligand receptor interactions trigger a rapid rise of cellular PIP3. Numerous molecular targets are activated upon interaction with PIP3. One such target is the Ser/Thr kinase Akt, which requires the action of phosphoinositide dependent kinases, another step for potential fine tuning. Akt subsequently inactivates glycogen-synthase-kinase 3 and the pro-apoptotic factor BAD. [22]. PIP3 also activates Btk, an essential protein for normal B lymphocyte development and function [23] along with dozens of other targets including centaurin, profiling, cytohesin, etc. which control[2]
Medical Implications
| |||||||||||
Additional 3D Structures
Solved Structures of PI3K
Class I PI3K
PI3K SH2 Domain
2iug, 2iuh, 2iui – Crystal Structure of PI3K nSH2 Domain with Peptides
1h9o – Crystal Structure of PI3K SH2 Domain with PDGFR Peptide
1fu5, 1fu6 – NMR structure of nSH2 Domain from PI3K
PI3K ISH2 Domain
3mtt – Crystal Structure of PI3K ISH2 Beta Crystal
3l4q – Crystal Structure of PI3K ISH2 in Influenza
2v1y – Crystal Structure of ISH2 in complex with ADB
PI3K SH3 Domain
3i5s, 3i5r – Crystal Structure of SH3 Domain in complex with peptide
2kt1 – Crystal Structure of SH3 Domain in p85 beta
1pht – Crystal Structure of PI3K Alpha SH3 Domain
1pks, 1pkt – Crystal Structure of PI3K SH3 Domain
p110 Subunit of PI3K
3lj3 – Crystal Structure of PI3K Gamma bound to Pyrrolopyridine-Benzofuran Inhibitor
3l54 – Crystal Structure of PI3K Gamma
3l13, 3l16, 3l17 – Crystal Structure of Pan-PI3-Kinase with Inhibitor
3l08 – Crystal Structure of PI3K Gamma bound to GSK2126458
3ibe – Crystal Structure of PI3K Gamma bound to Pyrazolopyrimidine Inhibitor
3hhm, 3hiz – Crystal Structure of p110 & NISH2
3ene – Crystal Structure of PI3K Gamma with inhibitor
3dpd – Crystal Structure of PI3K with oxazines inhibitor
3dbs – Crystal Structure of PI3K Gamma bound to GDC0941
3csf, 3cst – Crystal Structure of p110 Gamma bound to organourethenium inhibitor
2x38 – Crystal Structure of p110 Delta bound to IC87114
2wxf, 2wxg, 2wxh, 2wxi, 2wxj, 2wxk, 2wxl, 2wxm, 2wxn, 2wxo, 2wxp, 2wxq, 2wxr – Crystal Structure of p110 Delta with Inhibitors
2v4l – Crystal Structure of PI3K p110 Gamma with inhibitor
2rd0 – Crystal Structure of PI3K p110/p85 complex
2chw, 2chx, 2chz – Crystal Structure of PI3K Gamma with PIK-39 Inhibitor
2a4z, 2a5u – Crystal Structure of PI3K gamma complex with AS604850 and AS605240 Inhibitors
1he8 – Crystal Structure of RAS – PI3K Gamma Complex
1e7u, 1e7v, 1e7w, 1e7y, 1e7z, 1e90, 1e8x – Crystal Structure of PI3K Bound to Various Inhibitors
PI3K C2 Domain
2wwe – Crystal Structure of PI3K C2 Gamma Domain
2enq – Crystal Structure of C2 Domain, p110 Alpha
Class III PI3K
2x6f, 2x6h, 2x6j, 2x6k – Crystal Structure of Class III PI3K bound to various inhibitors
3ls8 – Crystal Structure of Class III PI3K in complex with inhibitor
3ihy – Crystal Structure of Human PI3K Class III
Additional Resources
- See: Cancer For Additional Proteins involved in the disease.
- See: Oncogenes for Additional examples of oncogenes and tumor suppressor genes.
References
- ↑ Djordjevic S, Driscoll PC. Structural insight into substrate specificity and regulatory mechanisms of phosphoinositide 3-kinases. Trends Biochem Sci. 2002 Aug;27(8):426-32. PMID:12151228
- ↑ 2.00 2.01 2.02 2.03 2.04 2.05 2.06 2.07 2.08 2.09 2.10 2.11 Wymann MP, Pirola L. Structure and function of phosphoinositide 3-kinases. Biochim Biophys Acta. 1998 Dec 8;1436(1-2):127-50. PMID:9838078
- ↑ 3.0 3.1 Sasaki T, Irie-Sasaki J, Horie Y, Bachmaier K, Fata JE, Li M, Suzuki A, Bouchard D, Ho A, Redston M, Gallinger S, Khokha R, Mak TW, Hawkins PT, Stephens L, Scherer SW, Tsao M, Penninger JM. Colorectal carcinomas in mice lacking the catalytic subunit of PI(3)Kgamma. Nature. 2000 Aug 24;406(6798):897-902. PMID:10972292 doi:10.1038/35022585
- ↑ Bi L, Okabe I, Bernard DJ, Wynshaw-Boris A, Nussbaum RL. Proliferative defect and embryonic lethality in mice homozygous for a deletion in the p110alpha subunit of phosphoinositide 3-kinase. J Biol Chem. 1999 Apr 16;274(16):10963-8. PMID:10196176
- ↑ 5.0 5.1 5.2 5.3 5.4 5.5 Miled N, Yan Y, Hon WC, Perisic O, Zvelebil M, Inbar Y, Schneidman-Duhovny D, Wolfson HJ, Backer JM, Williams RL. Mechanism of two classes of cancer mutations in the phosphoinositide 3-kinase catalytic subunit. Science. 2007 Jul 13;317(5835):239-42. PMID:17626883 doi:317/5835/239
- ↑ Stephens LR, Hughes KT, Irvine RF. Pathway of phosphatidylinositol(3,4,5)-trisphosphate synthesis in activated neutrophils. Nature. 1991 May 2;351(6321):33-9. PMID:1851250 doi:http://dx.doi.org/10.1038/351033a0
- ↑ 7.0 7.1 Hoedemaeker FJ, Siegal G, Roe SM, Driscoll PC, Abrahams JP. Crystal structure of the C-terminal SH2 domain of the p85alpha regulatory subunit of phosphoinositide 3-kinase: an SH2 domain mimicking its own substrate. J Mol Biol. 1999 Oct 1;292(4):763-70. PMID:10525402 doi:http://dx.doi.org/10.1006/jmbi.1999.3111
- ↑ Otsu M, Hiles I, Gout I, Fry MJ, Ruiz-Larrea F, Panayotou G, Thompson A, Dhand R, Hsuan J, Totty N, et al.. Characterization of two 85 kd proteins that associate with receptor tyrosine kinases, middle-T/pp60c-src complexes, and PI3-kinase. Cell. 1991 Apr 5;65(1):91-104. PMID:1707345
- ↑ 9.0 9.1 Batra-Safferling R, Granzin J, Modder S, Hoffmann S, Willbold D. Structural studies of the phosphatidylinositol 3-kinase (PI3K) SH3 domain in complex with a peptide ligand: role of the anchor residue in ligand binding. Biol Chem. 2010 Jan;391(1):33-42. PMID:19919182 doi:10.1515/BC.2010.003
- ↑ Koch CA, Anderson D, Moran MF, Ellis C, Pawson T. SH2 and SH3 domains: elements that control interactions of cytoplasmic signaling proteins. Science. 1991 May 3;252(5006):668-74. PMID:1708916
- ↑ Dombrosky-Ferlan PM, Corey SJ. Yeast two-hybrid in vivo association of the Src kinase Lyn with the proto-oncogene product Cbl but not with the p85 subunit of PI 3-kinase. Oncogene. 1997 May 1;14(17):2019-24. PMID:9160881 doi:10.1038/sj.onc.1201031
- ↑ Weber T, Schaffhausen B, Liu Y, Gunther UL. NMR structure of the N-SH2 of the p85 subunit of phosphoinositide 3-kinase complexed to a doubly phosphorylated peptide reveals a second phosphotyrosine binding site. Biochemistry. 2000 Dec 26;39(51):15860-9. PMID:11123912
- ↑ 13.0 13.1 13.2 13.3 13.4 13.5 13.6 13.7 Mandelker D, Gabelli SB, Schmidt-Kittler O, Zhu J, Cheong I, Huang CH, Kinzler KW, Vogelstein B, Amzel LM. A frequent kinase domain mutation that changes the interaction between PI3Kalpha and the membrane. Proc Natl Acad Sci U S A. 2009 Oct 6;106(40):16996-7001. Epub 2009 Sep 23. PMID:19805105
- ↑ Dhand R, Hiles I, Panayotou G, Roche S, Fry MJ, Gout I, Totty NF, Truong O, Vicendo P, Yonezawa K, et al.. PI 3-kinase is a dual specificity enzyme: autoregulation by an intrinsic protein-serine kinase activity. EMBO J. 1994 Feb 1;13(3):522-33. PMID:8313897
- ↑ von Willebrand M, Williams S, Saxena M, Gilman J, Tailor P, Jascur T, Amarante-Mendes GP, Green DR, Mustelin T. Modification of phosphatidylinositol 3-kinase SH2 domain binding properties by Abl- or Lck-mediated tyrosine phosphorylation at Tyr-688. J Biol Chem. 1998 Feb 13;273(7):3994-4000. PMID:9461588
- ↑ 16.0 16.1 Walker EH, Perisic O, Ried C, Stephens L, Williams RL. Structural insights into phosphoinositide 3-kinase catalysis and signalling. Nature. 1999 Nov 18;402(6759):313-20. PMID:10580505 doi:10.1038/46319
- ↑ Gout I, Dhand R, Hiles ID, Fry MJ, Panayotou G, Das P, Truong O, Totty NF, Hsuan J, Booker GW, et al.. The GTPase dynamin binds to and is activated by a subset of SH3 domains. Cell. 1993 Oct 8;75(1):25-36. PMID:8402898
- ↑ Wymann MP, Pirola L. Structure and function of phosphoinositide 3-kinases. Biochim Biophys Acta. 1998 Dec 8;1436(1-2):127-50. PMID:9838078
- ↑ 19.0 19.1 Stein RC. Prospects for phosphoinositide 3-kinase inhibition as a cancer treatment. Endocr Relat Cancer. 2001 Sep;8(3):237-48. PMID:11566615
- ↑ Sutton PR. Are most fluoridation promoters neurotics? Med Hypotheses. 1992 Nov;39(3):199-200. PMID:1474947
- ↑ 21.0 21.1 21.2 Walker EH, Pacold ME, Perisic O, Stephens L, Hawkins PT, Wymann MP, Williams RL. Structural determinants of phosphoinositide 3-kinase inhibition by wortmannin, LY294002, quercetin, myricetin, and staurosporine. Mol Cell. 2000 Oct;6(4):909-19. PMID:11090628
- ↑ Datta SR, Dudek H, Tao X, Masters S, Fu H, Gotoh Y, Greenberg ME. Akt phosphorylation of BAD couples survival signals to the cell-intrinsic death machinery. Cell. 1997 Oct 17;91(2):231-41. PMID:9346240
- ↑ de Weers M, Mensink RG, Kraakman ME, Schuurman RK, Hendriks RW. Mutation analysis of the Bruton's tyrosine kinase gene in X-linked agammaglobulinemia: identification of a mutation which affects the same codon as is altered in immunodeficient xid mice. Hum Mol Genet. 1994 Jan;3(1):161-6. PMID:8162018
- ↑ Lin RC, Weeks KL, Gao XM, Williams RB, Bernardo BC, Kiriazis H, Matthews VB, Woodcock EA, Bouwman RD, Mollica JP, Speirs HJ, Dawes IW, Daly RJ, Shioi T, Izumo S, Febbraio MA, Du XJ, McMullen JR. PI3K(p110 alpha) protects against myocardial infarction-induced heart failure: identification of PI3K-regulated miRNA and mRNA. Arterioscler Thromb Vasc Biol. 2010 Apr;30(4):724-32. PMID:20237330 doi:10.1161/ATVBAHA.109.201988
- ↑ Carracedo A, Pandolfi PP. The PTEN-PI3K pathway: of feedbacks and cross-talks. Oncogene. 2008 Sep 18;27(41):5527-41. PMID:18794886 doi:10.1038/onc.2008.247
- ↑ Crabbe T. Exploring the potential of PI3K inhibitors for inflammation and cancer. Biochem Soc Trans. 2007 Apr;35(Pt 2):253-6. PMID:17371252 doi:10.1042/BST0350253
Proteopedia Page Contributors and Editors (what is this?)
David Canner, Michal Harel, Joel L. Sussman, Jaime Prilusky, Hannah Campbell, Alexander Berchansky, Angel Herraez