Phosphoinositide 3-Kinases
From Proteopedia
| Line 8: | Line 8: | ||
PI3Ks are activated by extracellular agonists via the translocation of PI3Ks to the plasma membrane for easy access to lipid substrates. Depending on the adaptor proteins involved in the process, Class I PI3Ks are segregated into two subgroups. Those that associate with p85 will be directed to phosphorylated tyrosine motifs (Class IA), while PI3Kγ interacts with trimeric G proteins and the p101 protein (Class IB) <ref name="Wymann"/> | PI3Ks are activated by extracellular agonists via the translocation of PI3Ks to the plasma membrane for easy access to lipid substrates. Depending on the adaptor proteins involved in the process, Class I PI3Ks are segregated into two subgroups. Those that associate with p85 will be directed to phosphorylated tyrosine motifs (Class IA), while PI3Kγ interacts with trimeric G proteins and the p101 protein (Class IB) <ref name="Wymann"/> | ||
==Structure of PI3K== | ==Structure of PI3K== | ||
| - | Class I PI3Ks, which are tightly regulated by tyrosine kinases, are composed of an 85kDa regulatory/adapter subunit (p85) and a 110kDa catalytic subunit (p110). <ref name="Flip"> PMID: 10525402</ref> | + | Class I PI3Ks, which are tightly regulated by tyrosine kinases, are composed of an 85kDa regulatory/adapter subunit (p85) and a 110kDa catalytic subunit (p110). <ref name="Flip"> PMID: 10525402</ref> For More Information, See: [[The Structure of PI3K]] |
<br /> | <br /> | ||
| - | |||
| - | ==Adapter Subunit== | ||
| - | <StructureSection load='1dq8' size='500' side='left' scene='User:David_Canner/Sandbox_P/Full/4' caption='Structure of PI3K p110, ([[3hhm]])'> | ||
| - | ===The p85 Adapter Subunit=== | ||
| - | Class IA PI3Ks are tightly associated with a 85 kDa regulatory subunit called p85.<ref name="Wymann"/> P85 contains a Src homology 3 (SH3) domain, a breakpoint-cluster region homology (BH) domain between two proline-rich regions, and two C-terminal SH2 domains separated by an inter-SH2 (iSH2) region, which tightly binds p85 to the catalytic subunit.<ref>PMID:1707345</ref> Since PI3K has multiple protein-interaction domains, p85 is able to interact with several signaling molecules simultaneously, allowing for significant fine tuning of PI3K activity. <ref name="Wymann"/> | ||
| - | |||
| - | ====Src Homology 3 (SH3) Domain==== | ||
| - | <scene name='User:David_Canner/Sandbox_P/Sh3_network_open/1'>The SH3 domain of PI3K</scene> has homologues found in many intracellular signaling proteins. <ref name="Batra">PMID:19919182</ref> It mediates protein-protein interactions by binding to proline-rich motifs in target proteins forming multimeric signaling complexes. <ref>PMID:1708916</ref> Of note, the SH3 domain interacts with Src (Src homology 2/α-collagen-related), CDC42GAP (Cdc42 GTPase-activating protein) and the [[oncogene|proto-oncogene]] product Cbl. SH3 binds to proline rich ligands via a network of hydrophobic and hydrogen bond interactions, <scene name='User:David_Canner/Sandbox_P/Sh3_network/3'>particularly with the conserved residues Trp 55, Pro 70, and Tyr 73</scene> ([[3i5r]]).<ref name="Batra"/> | ||
| - | <br /> | ||
| - | |||
| - | ====Proline-Rich Regions==== | ||
| - | The proline rich regions which flank the BH domain are ideal ligands for various SH3 containing non-receptor protein tyrosine kinases like [[Src]], Lyn & Fyn, often with the product of the proteo-oncogene product Cbl as a docking site. <ref>PMID:9160881</ref> | ||
| - | <br /> | ||
| - | |||
| - | ====Src Homology 2 (SH2) Domains==== | ||
| - | <scene name='User:David_Canner/Sandbox_P/Sh2_open/1'>PI3K has two SH2 domains</scene>, an N-terminal (nSH2) domain and a C-terminal (CSH2) domain. <ref name="Flip"/> Both domains recognize similar consensus phosphorylated tyrosine motifs with the pattern: pY-V-X-M in activated receptors and adaptor proteins like PDGF, erbB3, c-Kit and CSF-1 receptors. <ref name="Weber">PMID:11123912</ref> It is upon the interaction of receptor and SH2 domain that the heterodimeric PI3K complex is activated. <ref name="Miled"> The <scene name='User:David_Canner/Sandbox_P/Sh2/2'>docking site for receptor in NSH2 is defined by the conserved residues Arg 340, Arg 358, and Thr 371</scene> ([[2iui]]), all of which coordinate the phosphorylated tyrosoine phosphate group.<ref name="Nolte"> PMID:8599763</ref> nSH2 was found to interact with the catalytic subunit directly, forming a broad-based scaffold for p110α and coordinates communication between the interacting domains. (Discussed Below). <ref name="Amzel"/> | ||
| - | <br /> | ||
| - | |||
| - | ====BH Domain==== | ||
| - | The BH domain specifically interacts with the Rho family proteins, Cdc42 and Rac1. Although no crystal structure of the BH domain has been solved to date, mutagenesis experiments have verified that the conserved residues Arg 151, Lys 187 and Pro 270 play important roles in the interaction with Rac1 and Cdc42. <ref name="Wymann"/> | ||
| - | <br /> | ||
| - | |||
| - | ====Inter-SH2 (iSH2) Region==== | ||
| - | The <scene name='User:David_Canner/Sandbox_P/Ish2/2'>iSH2, two long coiled alpha helices</scene> ([[2v1y]]), is flanked by the two <scene name='User:David_Canner/Sandbox_P/Sh2_flanked_ish2/1'>SH2 domains</scene>. The primary purpose of the iSH2 is to <scene name='User:David_Canner/Sandbox_P/Abd/3'>tightly bind the adaptor-binding domain (ABD) on the catalytic p110 subunit</scene> ([[3hhm]]), effectively holding the PI3K heterodimer together. In fact, disruption of this interaction via antibodies prevents the formation of the PI3K heterodimer completely. <ref name="Wymann"/> It is further believed that binding of phosphopetide by the SH2 domains causes conformational strains which is <scene name='User:David_Canner/Sandbox_P/Ish2_propagation/2'>propagated to the catalytic subunit directly and via the iSH2</scene><ref name="Wymann"/> | ||
| - | <br /> | ||
| - | |||
| - | ==Regulation of Class IA PI3K via p85 Phosphorylation== | ||
| - | All PI3K catalytic subunits possess intrinsic protein serine kinase activity. PI3K regulatory subunits can be phophorylated by the catalytic subunit (p110) at specific sites. For example, phophorylation of Ser 608, a residue located in an area of the iSH2 domain that is critical for PIP2 presentation to the catalytic subunit, results in a dramatic reduction in PI3K lipid kinase activity.<ref>PMID: 8313897</ref> Additionally, tyrosines 580 and 607 can be phosphorylated upon stimulation with insulin and growth factor along with <scene name='User:David_Canner/Sandbox_P/Tyr_508/1'>Tyr 508 upon PDGF receptor mediation</scene>. <ref name="Wymann"/> Phosphorylation of Tyr 688 in the CSH2 domain by Abl and Lck results in reduced affinity for phosphopeptides and subsequent activation of the catalytic domain. <ref>PMID:9461588 </ref> | ||
| - | <br /> | ||
| - | __NOTOC__</StructureSection> | ||
| - | |||
| - | ==The Catalytic Subunit== | ||
| - | <StructureSection load='1dq8' size='500' side='right' scene='User:David_Canner/Sandbox_P/Full/4' caption='Structure of PI3K p110, ([[3hhm]])'> | ||
| - | ===The Catalytic Subunit (P110) of Class 1 PI3Ks=== | ||
| - | The catalytic subunit, P110 has several isoforms that associate with different classes of PI3Ks. P110α, β, and δ associate with Class IA PI3Ks while p110γ associates with Class 1B PI3ks. <ref name="Wymann"/> The <scene name='User:David_Canner/Sandbox_P/Full/1'>p110α subunit contains several domains including</scene> an N-terminal adaptor-binding domain (ABD), a Ras binding domain (RBD) a C2 domain that likely binds to the cellular membrane, a helical domain (HD) with unknown function, and the actual catalytic kinase domain. <ref name="Amzel"> PMID: 19805105 </ref> The actions of these domains are coordinated by the nSH2 communicating domain in p85. | ||
| - | <br /> | ||
| - | |||
| - | ===Communication between nSH2 & The Catalytic Subunit Domains=== | ||
| - | <scene name='User:David_Canner/Sandbox_P/Nsh2_full/1'>The alpha-A helix of NSH2 </scene> (residues 340-345) is anchored into <scene name='User:David_Canner/Sandbox_P/Nsh2_pocket/2'> a cavity created by the C2 and Kinase domain interface.</scene> Helix α11K of the <scene name='User:David_Canner/Sandbox_P/Kinase_domain_out/2'>Kinase domain</scene> (residues 1017-1024) <scene name='User:David_Canner/Sandbox_P/Nsh2_kianse/1'>interacts with the alpha-A helix of nSH2.</scene> nSH2 interacts with the <scene name='User:David_Canner/Sandbox_P/C2_out/3'>C2 domain</scene> through a network of charge-charge interactions involving two loops on nSH2 (Residues 374-377 & 350-354) and C2 residues 364-371, a strong <scene name='User:David_Canner/Sandbox_P/Nsh2_charge_charge/3'>salt bridge between NSH2 Glu 349 and C2 residue Arg 357, and hydrogen bonds between NSH2 Glu 348 and C2 Glu 453 and Asp 454.</scene> <ref name="Amzel"/> | ||
| - | <br /> | ||
| - | |||
| - | The <scene name='User:David_Canner/Sandbox_P/Helical_overview/2'>helical domain in p110</scene>, whose function isn’t thoroughly understood, interacts with nSH2 via charge interactions. The HD residue, <scene name='User:David_Canner/Sandbox_P/Helical_domain/1'>Glu 542 forms a slat bridge with Arg 358 on NSH2 while Glu 545 interacts with NSH2 Lys 379</scene>. These residues are known hotspot mutations which are associated with various types of cancer. <ref name="Amzel"/> This loop in <scene name='User:David_Canner/Sandbox_P/Nsh2__and_helical_ligand_out/2'>the helical domain </scene> which contains the hotspots (residues 542-546) is located precisely where <scene name='User:David_Canner/Sandbox_P/Nsh2_ligand_just_ligand_full/1'> the phosphopeptide of NSH2 ligands, like PDGFR, bind to NSH2.</scene> The salt bridge formed between <scene name='User:David_Canner/Sandbox_P/Nsh2_disruption_of_salt/1'>Glu 542 and nSH2 is disrupted upon binding phosphorylated peptides</scene> like PDGFR, eliminating nSH2-mediated inhibition of p110α and activating the enzyme to phosphorylate PIP2 into PIP3. The hotspot mutation at Glu 542 accomplishes the same thing by eliminating the salt bridge and uninhibiting p110α. It is the <scene name='User:David_Canner/Sandbox_P/Kinase_with_atp_full/2'>kinase domain </scene> which <scene name='User:David_Canner/Sandbox_P/Kinase_with_atp_zoomed/3'>binds ATP to provide the phosphate group</scene> used to convert PIP2 into PIP3. <ref name="Amzel"/> | ||
| - | |||
| - | ===Model for Catalysis=== | ||
| - | Although no <scene name='User:David_Canner/Sandbox_P/Inhibitor_main/4'>crystal structure of PI3K</scene> with bound substate analog has been solved, a model for PIP2 phosphorylation has been developed and is generally supported. <ref name="Walker2">PMID:10580505</ref> In this model, the headgroup of PIP2 is <scene name='User:David_Canner/Sandbox_P/Catalytic_cavity/2'>positioned in a cavity</scene> between the <scene name='User:David_Canner/Sandbox_P/Catalytic_site/1'>C-terminal helix 12 of the kinase domain, the “activation” loop, and the “catalytic” loop</scene>. This puts the 5-phosphate of PIP2 near Lys 973 and the <scene name='User:David_Canner/Sandbox_P/Catalytic_site_atp_lys/1'>I-phosphate of ATP near Lys 807 and Lys 808</scene>. The <scene name='User:David_Canner/Sandbox_P/Catalytic_site_pip2/1'>basic residues Arg 947</scene> and Lys 973 can bind the 4-Phosphate of PIP2 and help provide the Class I PI3Ks with their specificity for PIP2. Once PIP2 and ATP are bound, it is believed <scene name='User:David_Canner/Sandbox_P/Catalytic_site_his/1'>His 948 rotates to interact with PIP2</scene>, deprotonating it at the C-3 Hydroxyl position creating a nucleophile. This nucleophile subsequently attacks the gamma phosphate of ATP producing PIP3. <ref name="Walker2"/> | ||
| - | </StructureSection> | ||
==Activation of Class IA PI3K== | ==Activation of Class IA PI3K== | ||
Revision as of 06:33, 15 November 2010
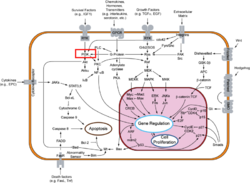
Template:STRUCTURE 3hhm Phosphoinositide 3-Kinases (PI3K) are a family of ubiquitously distributed lipid kinases, that play a critical role in the regulation of numerous cellular processes including cellular growth and morphology, programmed cell death, cell motility and adhesion, mitogenesis and glucose uptake. [1] PI3K generates important second messengers by catalyzing the transfer of the γ-phosphate group of ATP to the D3 position of phosphoinositides. [2] The PI3K preferred substrate is Phosphatidylinositol-4,5-bisphosphate (PIP2), which is converted into phosphatidylinositol-3,4,5-triphosphate (PIP3) upon phosphorylation at the cell membrane. The importance of PI3K is evident in knockout mice studies in which those mice with disruptions of critical PI3K components have significant deficiencies in immune and inflammatory response [3] sometimes resulting in embryonic death.[4] Aberrations in PIP3 levels, either through activation of PI3ks or through inactivation of lipid phosphatase PTEN, occur frequently in numerous forms of cancer, making PI3K an exciting new target to treat cancer among other human diseases.[5]
The Classes of PI3Ks
PI3Ks can be grouped into three distinct classes, Class I-III. Class I PI3Ks, the most well understood and thoroughly explored PI3K class, are composed of a 110kDa and a 50-100 kDa . Activation of Class I PI3Ks is controlled by extracellular signaling via receptors with intrinsic tyrosine kinase activity, G protein-linked receptors, or receptors coupled to SRC like protein tyrosine kinases. [6] Class II PI3Ks are relatively poorly understood but are 170-210 kDa and have in vitro substrate specificity toward PtdIns 4-P. Class III PI3Ks depend on Vps15p protein Ser/Thr kinases, which recruits the phosphatidylinositol kinase to late Golgi Compartments. [2]Class I Subclasses
PI3Ks are activated by extracellular agonists via the translocation of PI3Ks to the plasma membrane for easy access to lipid substrates. Depending on the adaptor proteins involved in the process, Class I PI3Ks are segregated into two subgroups. Those that associate with p85 will be directed to phosphorylated tyrosine motifs (Class IA), while PI3Kγ interacts with trimeric G proteins and the p101 protein (Class IB) [2]
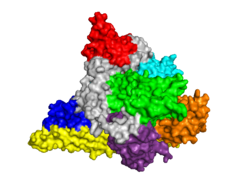
Structure of PI3K
Class I PI3Ks, which are tightly regulated by tyrosine kinases, are composed of an 85kDa regulatory/adapter subunit (p85) and a 110kDa catalytic subunit (p110). [7] For More Information, See: The Structure of PI3K
Activation of Class IA PI3K
Inactive PI3Ks are rapidly activated in the presence of extracellular stimuli. Such stimuli, as discussed previously, include growth factor receptors with intrinsic protein tyrosine kinase activity, which display pYXXM motifs for p85 docking, as well as receptor substrates which are phosphorylated and interact with PI3K regulatory subunits like nSH2. PI3K can be additionally activated in cooperative processes like translocation to the plasma membrane where lipid substrates are available and by binding GTP loaded Ras to the catalytic subunit. [8]. [2]
PI3K Inhibition
| |||||||||||
The Phosphorylated Lipid Products in Downstream Signaling
Ligand receptor interactions trigger a rapid rise of cellular PIP3. Numerous molecular targets are activated upon interaction with PIP3. One such target is the Ser/Thr kinase Akt, which requires the action of phosphoinositide dependent kinases, another step for potential fine tuning. Akt subsequently inactivates glycogen-synthase-kinase 3 and the pro-apoptotic factor BAD. [14]. PIP3 also activates Btk, an essential protein for normal B lymphocyte development and function [15] along with dozens of other targets including centaurin, profiling, cytohesin, etc. which control[2]
Medical Implications
| |||||||||||
Additional 3D Structures
Solved Structures of PI3K
Class I PI3K
PI3K SH2 Domain
2iug, 2iuh, 2iui – Crystal Structure of PI3K nSH2 Domain with Peptides
1h9o – Crystal Structure of PI3K SH2 Domain with PDGFR Peptide
1fu5, 1fu6 – NMR structure of nSH2 Domain from PI3K
PI3K ISH2 Domain
3mtt – Crystal Structure of PI3K ISH2 Beta Crystal
3l4q – Crystal Structure of PI3K ISH2 in Influenza
2v1y – Crystal Structure of ISH2 in complex with ADB
PI3K SH3 Domain
3i5s, 3i5r – Crystal Structure of SH3 Domain in complex with peptide
2kt1 – Crystal Structure of SH3 Domain in p85 beta
1pht – Crystal Structure of PI3K Alpha SH3 Domain
1pks, 1pkt – Crystal Structure of PI3K SH3 Domain
p110 Subunit of PI3K
3lj3 – Crystal Structure of PI3K Gamma bound to Pyrrolopyridine-Benzofuran Inhibitor
3l54 – Crystal Structure of PI3K Gamma
3l13, 3l16, 3l17 – Crystal Structure of Pan-PI3-Kinase with Inhibitor
3l08 – Crystal Structure of PI3K Gamma bound to GSK2126458
3ibe – Crystal Structure of PI3K Gamma bound to Pyrazolopyrimidine Inhibitor
3hhm, 3hiz – Crystal Structure of p110 & NISH2
3ene – Crystal Structure of PI3K Gamma with inhibitor
3dpd – Crystal Structure of PI3K with oxazines inhibitor
3dbs – Crystal Structure of PI3K Gamma bound to GDC0941
3csf, 3cst – Crystal Structure of p110 Gamma bound to organourethenium inhibitor
2x38 – Crystal Structure of p110 Delta bound to IC87114
2wxf, 2wxg, 2wxh, 2wxi, 2wxj, 2wxk, 2wxl, 2wxm, 2wxn, 2wxo, 2wxp, 2wxq, 2wxr – Crystal Structure of p110 Delta with Inhibitors
2v4l – Crystal Structure of PI3K p110 Gamma with inhibitor
2rd0 – Crystal Structure of PI3K p110/p85 complex
2chw, 2chx, 2chz – Crystal Structure of PI3K Gamma with PIK-39 Inhibitor
2a4z, 2a5u – Crystal Structure of PI3K gamma complex with AS604850 and AS605240 Inhibitors
1he8 – Crystal Structure of RAS – PI3K Gamma Complex
1e7u, 1e7v, 1e7w, 1e7y, 1e7z, 1e90, 1e8x – Crystal Structure of PI3K Bound to Various Inhibitors
PI3K C2 Domain
2wwe – Crystal Structure of PI3K C2 Gamma Domain
2enq – Crystal Structure of C2 Domain, p110 Alpha
Class III PI3K
2x6f, 2x6h, 2x6j, 2x6k – Crystal Structure of Class III PI3K bound to various inhibitors
3ls8 – Crystal Structure of Class III PI3K in complex with inhibitor
3ihy – Crystal Structure of Human PI3K Class III
Additional Resources
- See: Cancer For Additional Proteins involved in the disease.
- See: Oncogenes for Additional examples of oncogenes and tumor suppressor genes.
References
- ↑ Djordjevic S, Driscoll PC. Structural insight into substrate specificity and regulatory mechanisms of phosphoinositide 3-kinases. Trends Biochem Sci. 2002 Aug;27(8):426-32. PMID:12151228
- ↑ 2.0 2.1 2.2 2.3 2.4 Wymann MP, Pirola L. Structure and function of phosphoinositide 3-kinases. Biochim Biophys Acta. 1998 Dec 8;1436(1-2):127-50. PMID:9838078
- ↑ 3.0 3.1 Sasaki T, Irie-Sasaki J, Horie Y, Bachmaier K, Fata JE, Li M, Suzuki A, Bouchard D, Ho A, Redston M, Gallinger S, Khokha R, Mak TW, Hawkins PT, Stephens L, Scherer SW, Tsao M, Penninger JM. Colorectal carcinomas in mice lacking the catalytic subunit of PI(3)Kgamma. Nature. 2000 Aug 24;406(6798):897-902. PMID:10972292 doi:10.1038/35022585
- ↑ Bi L, Okabe I, Bernard DJ, Wynshaw-Boris A, Nussbaum RL. Proliferative defect and embryonic lethality in mice homozygous for a deletion in the p110alpha subunit of phosphoinositide 3-kinase. J Biol Chem. 1999 Apr 16;274(16):10963-8. PMID:10196176
- ↑ 5.0 5.1 5.2 5.3 5.4 Miled N, Yan Y, Hon WC, Perisic O, Zvelebil M, Inbar Y, Schneidman-Duhovny D, Wolfson HJ, Backer JM, Williams RL. Mechanism of two classes of cancer mutations in the phosphoinositide 3-kinase catalytic subunit. Science. 2007 Jul 13;317(5835):239-42. PMID:17626883 doi:317/5835/239
- ↑ Stephens LR, Hughes KT, Irvine RF. Pathway of phosphatidylinositol(3,4,5)-trisphosphate synthesis in activated neutrophils. Nature. 1991 May 2;351(6321):33-9. PMID:1851250 doi:http://dx.doi.org/10.1038/351033a0
- ↑ Hoedemaeker FJ, Siegal G, Roe SM, Driscoll PC, Abrahams JP. Crystal structure of the C-terminal SH2 domain of the p85alpha regulatory subunit of phosphoinositide 3-kinase: an SH2 domain mimicking its own substrate. J Mol Biol. 1999 Oct 1;292(4):763-70. PMID:10525402 doi:http://dx.doi.org/10.1006/jmbi.1999.3111
- ↑ Gout I, Dhand R, Hiles ID, Fry MJ, Panayotou G, Das P, Truong O, Totty NF, Hsuan J, Booker GW, et al.. The GTPase dynamin binds to and is activated by a subset of SH3 domains. Cell. 1993 Oct 8;75(1):25-36. PMID:8402898
- ↑ Wymann MP, Pirola L. Structure and function of phosphoinositide 3-kinases. Biochim Biophys Acta. 1998 Dec 8;1436(1-2):127-50. PMID:9838078
- ↑ 10.0 10.1 Stein RC. Prospects for phosphoinositide 3-kinase inhibition as a cancer treatment. Endocr Relat Cancer. 2001 Sep;8(3):237-48. PMID:11566615
- ↑ Sutton PR. Are most fluoridation promoters neurotics? Med Hypotheses. 1992 Nov;39(3):199-200. PMID:1474947
- ↑ Cite error: Invalid
<ref>tag; no text was provided for refs namedAmzel - ↑ 13.0 13.1 13.2 Walker EH, Pacold ME, Perisic O, Stephens L, Hawkins PT, Wymann MP, Williams RL. Structural determinants of phosphoinositide 3-kinase inhibition by wortmannin, LY294002, quercetin, myricetin, and staurosporine. Mol Cell. 2000 Oct;6(4):909-19. PMID:11090628
- ↑ Datta SR, Dudek H, Tao X, Masters S, Fu H, Gotoh Y, Greenberg ME. Akt phosphorylation of BAD couples survival signals to the cell-intrinsic death machinery. Cell. 1997 Oct 17;91(2):231-41. PMID:9346240
- ↑ de Weers M, Mensink RG, Kraakman ME, Schuurman RK, Hendriks RW. Mutation analysis of the Bruton's tyrosine kinase gene in X-linked agammaglobulinemia: identification of a mutation which affects the same codon as is altered in immunodeficient xid mice. Hum Mol Genet. 1994 Jan;3(1):161-6. PMID:8162018
- ↑ Lin RC, Weeks KL, Gao XM, Williams RB, Bernardo BC, Kiriazis H, Matthews VB, Woodcock EA, Bouwman RD, Mollica JP, Speirs HJ, Dawes IW, Daly RJ, Shioi T, Izumo S, Febbraio MA, Du XJ, McMullen JR. PI3K(p110 alpha) protects against myocardial infarction-induced heart failure: identification of PI3K-regulated miRNA and mRNA. Arterioscler Thromb Vasc Biol. 2010 Apr;30(4):724-32. PMID:20237330 doi:10.1161/ATVBAHA.109.201988
- ↑ Carracedo A, Pandolfi PP. The PTEN-PI3K pathway: of feedbacks and cross-talks. Oncogene. 2008 Sep 18;27(41):5527-41. PMID:18794886 doi:10.1038/onc.2008.247
- ↑ Crabbe T. Exploring the potential of PI3K inhibitors for inflammation and cancer. Biochem Soc Trans. 2007 Apr;35(Pt 2):253-6. PMID:17371252 doi:10.1042/BST0350253
Proteopedia Page Contributors and Editors (what is this?)
David Canner, Michal Harel, Joel L. Sussman, Jaime Prilusky, Hannah Campbell, Alexander Berchansky, Angel Herraez