Aquaporin
From Proteopedia
| Line 54: | Line 54: | ||
| - | 1. Crane | + | 1. Crane, J.M., Tajima, M., and Verkman, A.S. Live-cell imaging of aquaporin-4 diffusion and interactions in orthogonal arrays of particles. <i>Neuroscience</i> (2010) vol. 168 (4) pp. 892-902<br/> |
| - | 2. Hiroaki | + | 2. Hiroaki, Y., Tani, K., Kamegawa, A., Gyobu, N., Nishikawa, K., Suzuki, H., Walz, T., Sasaki, S., Mitsuoka, Kimura, K., Mizoguchi, A., and Fujiyoshi, Y. Implications of the aquaporin-4 structure on array formation and cell adhesion. <i>J Mol Biol</i> (2006) vol. 355 (4) pp. 628-39<br/> |
| - | 3. Nicchia | + | 3. Nicchia, G.P., Rossi, A., Mola, M.G., Pisani, F., Stigliano, C., Basco, D., Mastrototaro, M., Svelto, M., and A. Frigeri. Higher order structure of aquaporin-4. <i>Neuroscience</i> (2010) vol. 168 (4) pp. 903-14<br/> |
| - | 4. Pittock SJ, Lennon | + | 4. Pittock SJ, and Lennon V.A. Aquaporin-4 autoantibodies in a paraneoplastic context. <i>Arch Neurol</i> (2008) 65:629–632.<br/> |
| - | 5. Neurological | + | 5. Hinson, S.R., McKeon, A., and Lennon V.A. Neurological autoimmunity targeting aquaporin-4. <i>Neuroscience</i> (2010) vol. 168 (4) pp. 1009-18.<br/> |
| - | 6. Saadoun S, Papadopoulos | + | 6. Saadoun S., Papadopoulos M.C., Hara-Chikuma M, Verkman A.S. Impairment of angiogenesis and cell migration by targeted aquaporin-1 gene disruption. <i>Nature</i> (2005) 434:786–792.<br/> |
| - | 7. Verkman | + | 7. Verkman A.S., Hara-Chikuma M., Papadopoulos M.C. Aquaporins—new players in cancer biology. <i>J Mol Med</i> (2008) 86:523–529. |
Revision as of 00:25, 30 November 2010
| |||||||||
| Human Aquaporin 4, 3gd8 | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Ligands: | , | ||||||||
| Gene: | AQP4 (Homo sapiens) | ||||||||
| |||||||||
| |||||||||
| Resources: | FirstGlance, OCA, RCSB, PDBsum | ||||||||
| Coordinates: | save as pdb, mmCIF, xml | ||||||||
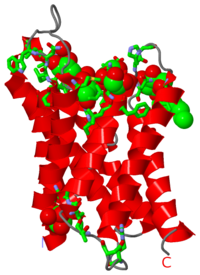
Aquaporins are channel producing proteins which regulate the flow of water across the cell membrane. They are made of α-helix bundles. Aquaglyceroporin (GLpf) conducts water and polyalcohols. The images at the left and at the right correspond to one representative aquaporin structure, i.e. the crystal structure of human aquaporin 4 (3gd8). The image shows the protein, 6 molecules of glycerol and one of beta-octylglucoside.
Contents |
3D Structures of Aquaporin
3llq – Aqp – Agrobacterium tumefaciens
2w1p, 2w2e – Aqp1 – Pischia pastoris
2zz9 – rAqp4 (mutant) – rat
2d57 – rAqp4 – electron crystallography
3gd8 – hAqp4 – human
3d9s – hAqp5
1h6i, 1ih5 – hAqp1
3cll, 3cn5, 3cn6 – sAqp SoPIP2 (mutant) – spinach
1z98, 2b5f - sAqp SoPIP2
2o9d, 2o9f – EcAqpZ (mutant) – Escherichia coli
2o9e, 2o9g - EcAqpZ (mutant)+Hg
2abm, 1rc2 - EcAqpZ
2c32, 1ymg – cAqp0 – cow
1j4n – cAqp1
2b6o – Aqp0 - electron crystallography – sheep
2evu, 2f2b – AqpM – Methanothermobacter marburgensis
3D Structures of Aquaglyceroporin
1lda, 1ldi – EcGLpf
1ldf – EcGLpf (mutant)
3c02 – GLpf – Plasmodium falciparum
Summary of AQP4 Function
AQP4 is the most abundant of the of three water channels in the Central Nervous System (CNS), and its expression is upregulated in the astrocytes of the brain cortex and spinal cord [5]. AQP4 allows only water to pass through the channel, preventing the transport of any solute [4]. It also plays a role in the removal of “excess brain water in vasogenic brain edema and hydrocephalus” by an unknown mechanism of bulk excretion of water [2]. However, AQP4 “provides a major route for water entry into the brain through an intact blood–brain barrier” [2], which exacerbates cytotoxic brain edema [2]. In addition, due to AQP4’s short extracellular loop formed from a 310 helix, AQP4 is able to play a role in cell adhesion by interacting with other AQP4 molecules on adjacent cell membranes. Finally, AQP4 plays a role in cell migration. For example, glial-scarring was reduced in the brain following injury due to AQP4-facilitated migration [9], and a role in brain tumor metastases is hypothesized because of AQP4’s elevated expression in glioblastomas and the role of AQP proteins in tumor cell migration [10].
Higher Order Structure of AQP4
AQP4 is organized in the plasma membrane into structures called orthogonal arrays of particles (OAPs). These structures are comprised of both the M23 and M1 isoforms of AQP4, though M23 is specifically required for the formation of OAPs as the amino acid sequence of the N-terminus of the M1 isoform appears to prevent formation of OAPs [4]. The recognition of AQP4’s role in the formation and structure of OAPs has led to further understanding in several neuromuscular diseases, including Duchenne Muscular Dystrophy, and NMO (see “Role in Autoimmune Disorders” below). There is also evidence that the formation of OAPs is physiologically regulated [5].
Role in Autoimmune Disorder
Clinical presentations of central nervous system (CNS) aquaporin-4 autoimmunity are consistent with neuromyelitis optica (NMO), and can include blindness and paraplegia. Previously, variants of the NMO phenotype were classified as differential presentations of Multiple Sclerosis (MS). The specific causes of CNS AQP4 autoimmunity are unknown. However, observations of two patients with brain metastases suggest that CNS AQP4 autoimmunity may develop as part of an immune response to cancer [7]. As is the case in many autoimmune disorders, treatment of CNS AQP4 autoimmunity with anti-inflammatories (such as corticosteroids) and antibody-depleting therapeutics (such as plasma exchange) have been effective at reducing symptoms. Therapies for NMO based on the role of CNS AQP4 autoimmunity await further experimentation of the disease model and cell culture systems. [8]
References
1. Crane, J.M., Tajima, M., and Verkman, A.S. Live-cell imaging of aquaporin-4 diffusion and interactions in orthogonal arrays of particles. Neuroscience (2010) vol. 168 (4) pp. 892-902
2. Hiroaki, Y., Tani, K., Kamegawa, A., Gyobu, N., Nishikawa, K., Suzuki, H., Walz, T., Sasaki, S., Mitsuoka, Kimura, K., Mizoguchi, A., and Fujiyoshi, Y. Implications of the aquaporin-4 structure on array formation and cell adhesion. J Mol Biol (2006) vol. 355 (4) pp. 628-39
3. Nicchia, G.P., Rossi, A., Mola, M.G., Pisani, F., Stigliano, C., Basco, D., Mastrototaro, M., Svelto, M., and A. Frigeri. Higher order structure of aquaporin-4. Neuroscience (2010) vol. 168 (4) pp. 903-14
4. Pittock SJ, and Lennon V.A. Aquaporin-4 autoantibodies in a paraneoplastic context. Arch Neurol (2008) 65:629–632.
5. Hinson, S.R., McKeon, A., and Lennon V.A. Neurological autoimmunity targeting aquaporin-4. Neuroscience (2010) vol. 168 (4) pp. 1009-18.
6. Saadoun S., Papadopoulos M.C., Hara-Chikuma M, Verkman A.S. Impairment of angiogenesis and cell migration by targeted aquaporin-1 gene disruption. Nature (2005) 434:786–792.
7. Verkman A.S., Hara-Chikuma M., Papadopoulos M.C. Aquaporins—new players in cancer biology. J Mol Med (2008) 86:523–529.
Proteopedia Page Contributors and Editors (what is this?)
Michal Harel, Alexander Berchansky, Mark Leiserson, David Canner, Jaime Prilusky, Eran Hodis

