We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
Cyclooxygenase
From Proteopedia
(Difference between revisions)
| Line 1: | Line 1: | ||
| - | + | ==Structure== | |
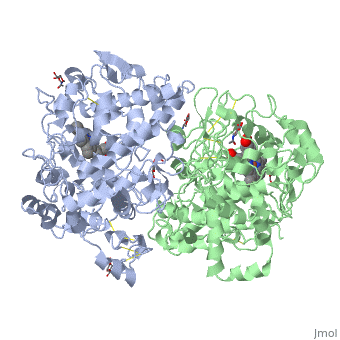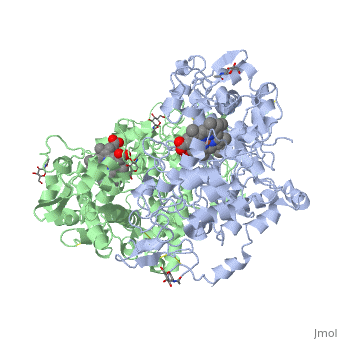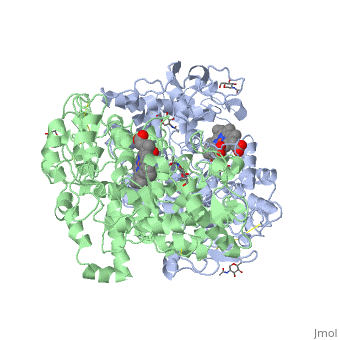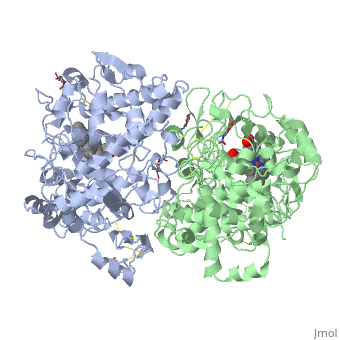
| - | + | <Structure load='5cox' size='400' frame='true' align='right' caption='Unhibited mouse cyclooxygenase-2' scene='Insert optional scene name here' /> | |
| - | + | ||
| - | < | + | |
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | === | + | |
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
==NSAIDs== | ==NSAIDs== | ||
{| border="1" style="margin: 1em auto 1em auto;" | {| border="1" style="margin: 1em auto 1em auto;" | ||
Revision as of 09:48, 7 December 2010
Structure
|
NSAIDs
| Drug | Coefficient of selectivity (IC50Cox-1/IC50Cox-2) |
|---|---|
| Ketorolac | |
| Naproxen | |
| Ibuprofen | |
| Indometacin | |
| Acetylsalicylic acid | |
| Diclofenac | |
| Valdecoxib | |
| Etoricoxib | |
| Pharmacologic group | Drug |
|---|---|
| Salicylates | Acetylsalicylic acid |
| Propionic | Naproxen |
| Ibuprofen | |
| Para-aminophenols | Paracetamol |
| Indolacetic | Indometacin |
| Pirrolacetic | Ketorolac |
| Phenilacetic | Diclofenac |
| Piranoidacetic | Etodolac |
| Anthranilic | Mefenamic acid |
| Nicotinic | Clonixin |
| Sulfonanilides | Nimesulide |
Reference
- Salom D, Lodowski DT, Stenkamp RE, Le Trong I, Golczak M, Jastrzebska B, Harris T, Ballesteros JA, Palczewski K. Crystal structure of a photoactivated deprotonated intermediate of rhodopsin. Proc Natl Acad Sci U S A. 2006 Oct 31;103(44):16123-8. Epub 2006 Oct 23. PMID:17060607
Page seeded by OCA on Tue Feb 17 02:59:53 2009
- Ghosh N, Chaki R, Mandal V, Mandal SC. COX-2 as a target for cancer chemotherapy. Pharmacol Rep. 2010 Mar-Apr;62(2):233-44. PMID:20508278
- Smith WL, DeWitt DL, Garavito RM. Cyclooxygenases: structural, cellular, and molecular biology. Annu Rev Biochem. 2000;69:145-82. PMID:10966456 doi:10.1146/annurev.biochem.69.1.145
- Rang HP, Dale MM, Ritter JM, Flower RJ. 2008. Pharmacology. Elsevier. 6th edition. 844 p.
- Smith WL, Langenbach R. Why there are two cyclooxygenase isozymes. J Clin Invest. 2001 Jun;107(12):1491-5. PMID:11413152 doi:10.1172/JCI13271
- Chandrasekharan NV, Dai H, Roos KL, Evanson NK, Tomsik J, Elton TS, Simmons DL. COX-3, a cyclooxygenase-1 variant inhibited by acetaminophen and other analgesic/antipyretic drugs: cloning, structure, and expression. Proc Natl Acad Sci U S A. 2002 Oct 15;99(21):13926-31. Epub 2002 Sep 19. PMID:12242329 doi:10.1073/pnas.162468699
Proteopedia Page Contributors and Editors (what is this?)
Cristina Murga, Michal Harel, David Canner, María Laura Saiz Álvarez, Alexander Berchansky