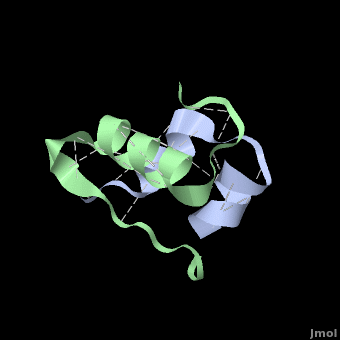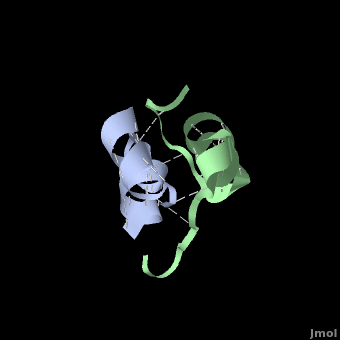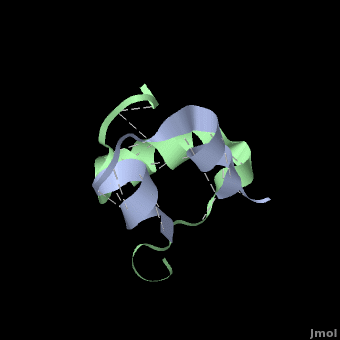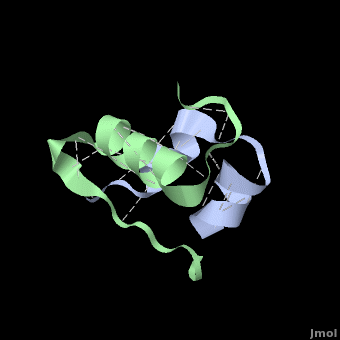Molecular Playground/Insulin
From Proteopedia
(Difference between revisions)
| Line 1: | Line 1: | ||
One of the [[CBI Molecules]] being studied in the [http://www.umass.edu/cbi/ University of Massachusetts Amherst Chemistry-Biology Interface Program] at UMass Amherst in the [http://robertsgroup.ecs.umass.edu/ Roberts Research Group] and on display at the [http://www.molecularplayground.org Molecular Playground]. | One of the [[CBI Molecules]] being studied in the [http://www.umass.edu/cbi/ University of Massachusetts Amherst Chemistry-Biology Interface Program] at UMass Amherst in the [http://robertsgroup.ecs.umass.edu/ Roberts Research Group] and on display at the [http://www.molecularplayground.org Molecular Playground]. | ||
| - | <applet size='[450,338]' frame='true' align='right' scene='User:Whitney_Stoppel/sandbox1/Human_insulin2/1 | + | <applet size='[450,338]' frame='true' align='right' scene='' 'caption='' /> |
| + | <StructureSection load='' size='500' side='right' scene='User:Whitney_Stoppel/sandbox1/Human_insulin2/1' caption='Insulin'> | ||
'''Insulin''' is a hormone that controls [[Carbohydrate Metabolism|carbohydrate metabolism]] and storage in the human body. The body is able to sense the concentration of glucose in the blood and respond by secreting insulin, which is produced by beta cells in the pancreas. Synthesis of human insulin in E. coli is important to producing insulin for the treatment of type 1 diabetes. | '''Insulin''' is a hormone that controls [[Carbohydrate Metabolism|carbohydrate metabolism]] and storage in the human body. The body is able to sense the concentration of glucose in the blood and respond by secreting insulin, which is produced by beta cells in the pancreas. Synthesis of human insulin in E. coli is important to producing insulin for the treatment of type 1 diabetes. | ||
| Line 7: | Line 8: | ||
Insulin is able to pair-up with itself and form a dimer by forming hydrogen bonds between the ends of two B-chains. These <scene name='User:Whitney_Stoppel/sandbox1/Insulin_dimer/2'>hydrogen bonds</scene> are shown above in white. Then, 3 dimers can come together in the presence of zinc ions and form a hexamer. Insulin is stored in the <scene name='User:Whitney_Stoppel/sandbox1/Insulin_hexamer/4'>hexameric form</scene> in the body. This <scene name='User:Whitney_Stoppel/sandbox1/Insulin_ph7/2'>scene highlights</scene> the hydrophobic (gray) and polar (purple) parts of an insulin monomer at a pH of 7. It is believed that the hydrophobic sections on the B-chain cause insulin aggregation which initially caused problems in the manufacture and storage of insulin for pharmaceutical use. | Insulin is able to pair-up with itself and form a dimer by forming hydrogen bonds between the ends of two B-chains. These <scene name='User:Whitney_Stoppel/sandbox1/Insulin_dimer/2'>hydrogen bonds</scene> are shown above in white. Then, 3 dimers can come together in the presence of zinc ions and form a hexamer. Insulin is stored in the <scene name='User:Whitney_Stoppel/sandbox1/Insulin_hexamer/4'>hexameric form</scene> in the body. This <scene name='User:Whitney_Stoppel/sandbox1/Insulin_ph7/2'>scene highlights</scene> the hydrophobic (gray) and polar (purple) parts of an insulin monomer at a pH of 7. It is believed that the hydrophobic sections on the B-chain cause insulin aggregation which initially caused problems in the manufacture and storage of insulin for pharmaceutical use. | ||
| - | + | </StructureSection> | |
==Additional Resources== | ==Additional Resources== | ||
For additional information, see: [[Diabetes & Hypoglycemia]] | For additional information, see: [[Diabetes & Hypoglycemia]] | ||
<br /> | <br /> | ||
Revision as of 10:18, 8 December 2010
One of the CBI Molecules being studied in the University of Massachusetts Amherst Chemistry-Biology Interface Program at UMass Amherst in the Roberts Research Group and on display at the Molecular Playground.
|
| |||||||||||
Additional Resources
For additional information, see: Diabetes & Hypoglycemia
Proteopedia Page Contributors and Editors (what is this?)
Michal Harel, Joel L. Sussman, Shelly Livne, Karsten Theis, David Canner, Whitney Stoppel, Alexander Berchansky, Yael Shwartz, Lynmarie K Thompson, Jaime Prilusky