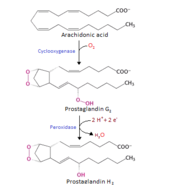
Cyclooxygenase
From Proteopedia
| Line 1: | Line 1: | ||
| - | '''COX-2''' | ||
| - | |||
COX-1 and COX-2, also called PGHS-1 and PGHS-2, regulate a key step in prostaglandin and thromboxane synthesis and are the targets of nonsteroidal antiinflammatory drugs (NSAIDs) <ref name="Smith&Langenbach2001">PMID: 11413152</ref> <ref name="Chandrasekharan2002">PMID: 12242329</ref> <ref name="Ghosh2010">PMID: 20508278</ref>. Prostaglandins are implicated in various pathophysiological processes such as inflammatory reactions, gastrointestinal cytoprotection, hemostasis and thrombosis, as well as renal hemodynamics <ref name="Smith&Langenbach2001" /> <ref name="Ghosh2010"/> <ref name="Smith2000">PMID: 10966456</ref>. Whereas COX-1 presents a widespread constitutive expression, COX-2 is undetectable in most normal tissues (except for the central nervous system, kidneys, and seminal vesicles), but is induced by various inflammatory and mitogenic stimuli <ref name="Smith2000" /> <ref name="Ghosh2010"/> <ref name="Rang&Dale2008">Rang HP, Dale MM, Ritter JM, Flower RJ. 2008. Pharmacology. Elsevier. 6th edition. UK. 844 p.</ref>. More recently, a third isoform named COX-3 was identified as a COX-1 splicing variant. This new isoform may play a role in processes such as fever and pain <ref name="Ghosh2010"/> <ref name="Chandrasekharan2002"/>. | COX-1 and COX-2, also called PGHS-1 and PGHS-2, regulate a key step in prostaglandin and thromboxane synthesis and are the targets of nonsteroidal antiinflammatory drugs (NSAIDs) <ref name="Smith&Langenbach2001">PMID: 11413152</ref> <ref name="Chandrasekharan2002">PMID: 12242329</ref> <ref name="Ghosh2010">PMID: 20508278</ref>. Prostaglandins are implicated in various pathophysiological processes such as inflammatory reactions, gastrointestinal cytoprotection, hemostasis and thrombosis, as well as renal hemodynamics <ref name="Smith&Langenbach2001" /> <ref name="Ghosh2010"/> <ref name="Smith2000">PMID: 10966456</ref>. Whereas COX-1 presents a widespread constitutive expression, COX-2 is undetectable in most normal tissues (except for the central nervous system, kidneys, and seminal vesicles), but is induced by various inflammatory and mitogenic stimuli <ref name="Smith2000" /> <ref name="Ghosh2010"/> <ref name="Rang&Dale2008">Rang HP, Dale MM, Ritter JM, Flower RJ. 2008. Pharmacology. Elsevier. 6th edition. UK. 844 p.</ref>. More recently, a third isoform named COX-3 was identified as a COX-1 splicing variant. This new isoform may play a role in processes such as fever and pain <ref name="Ghosh2010"/> <ref name="Chandrasekharan2002"/>. | ||
| - | Additionally, a high level of COX-2 expression is | + | Additionally, a high level of COX-2 expression is found usually in cancer cells <ref name="Ghosh2010"/>. For example, COX-2 overexpression is related to poor prognosis in certain breast cancers <ref name="Barnes2007">PMID: 17285134</ref> <ref name="Boland2004">PMID: 14735188</ref> and endometrial adenocarcinomas <ref name="Sales2008">PMID: 18316157</ref>. |
==Function== | ==Function== | ||
| Line 15: | Line 13: | ||
In 1994, Picot et al. published the first three-dimensional (3D) structure of a COX enzyme, the ovine COX-1 complexed with the NSAID flurbiprofen. Soon afterward, the crystal structures of human and murine COX-2 followed. First, the three-dimensional structure of human COX-2 was assessed by means of sequence homology modeling, but in 1996, Luong, C. ''et al'' <ref name="Luong1996">PMID: 8901870</ref> and Kurumbail, R.G ''et al'' <ref name="Kurumbail1996">PMID: 8967954</ref> published two crystal structures of the recombinant human and mouse COX-2 isozymes, respectively, complexed with different selective inhibitors. Given its pharmacological importance as a therepeutic target, drug interactions with COX were one of the first issues to be addressed, and complexes containing a number of different NSAIDs have been studied crystallographically. The structural analysis of COX complexed with substrates or products was more difficult to pursue for a number of technical reasons. However, within the past years, crystal structures of murine COX-2 complexed with AA and EPA have also been determined. | In 1994, Picot et al. published the first three-dimensional (3D) structure of a COX enzyme, the ovine COX-1 complexed with the NSAID flurbiprofen. Soon afterward, the crystal structures of human and murine COX-2 followed. First, the three-dimensional structure of human COX-2 was assessed by means of sequence homology modeling, but in 1996, Luong, C. ''et al'' <ref name="Luong1996">PMID: 8901870</ref> and Kurumbail, R.G ''et al'' <ref name="Kurumbail1996">PMID: 8967954</ref> published two crystal structures of the recombinant human and mouse COX-2 isozymes, respectively, complexed with different selective inhibitors. Given its pharmacological importance as a therepeutic target, drug interactions with COX were one of the first issues to be addressed, and complexes containing a number of different NSAIDs have been studied crystallographically. The structural analysis of COX complexed with substrates or products was more difficult to pursue for a number of technical reasons. However, within the past years, crystal structures of murine COX-2 complexed with AA and EPA have also been determined. | ||
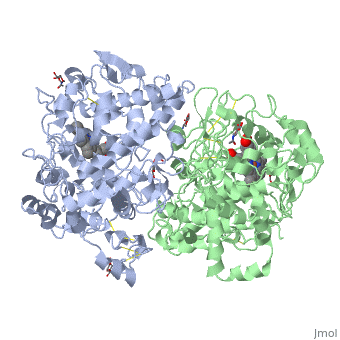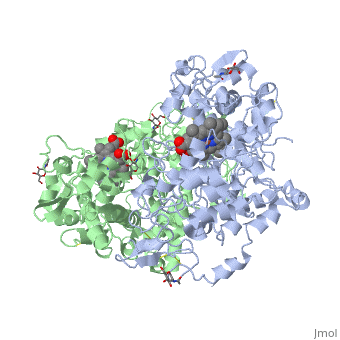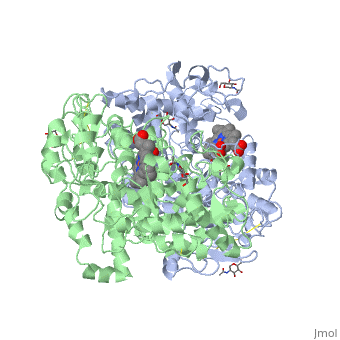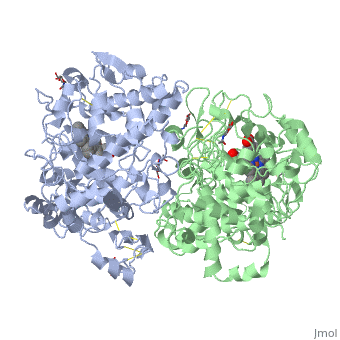
| - | PGHSs are bifunctional <scene name='SandboxUAM/Mynewscene/22'>homodimers</scene>. Both COX-1 and COX-2 are membrane-bound enzymes and are present on the luminal surfaces of the endoplasmic reticulum and of the inner and outer membranes of the nuclear envelope. | + | PGHSs are bifunctional <scene name='SandboxUAM/Mynewscene/22'>homodimers</scene>. Both COX-1 and COX-2 are membrane-bound enzymes and are present on the luminal surfaces of the endoplasmic reticulum and of the inner and outer membranes of the nuclear envelope. However, recently, using cultured endothelial cells and fibroblasts a fraction of COX-2 protein was shown to be localized to plasma membrane in caveolae-like structures <ref name="Perrone2007">PMID: 10551860</ref>. The primary structure of nascent COX-2 is of 604 amino acids and it is processed into a mature form by removal of signal peptides giving a protein of 587 amino acids. PGHS-2 is variably glycosylated at two to four sites, leading to the formation of doublets or sometimes triplets that can be detected on SDS-PAGE. In murine PGHS-2 <scene name='SandboxUAM/Mynewscene/8'>carbohydrate moieties</scene> are linked to Asn-68, Asn-144, and Asn-410 in each monomer <ref name="Vecchio2010">PMID: 20463020</ref>. |
The COX monomer consists of <scene name='SandboxUAM/Mynewscene/12'>three structural domains</scene>: the N-terminal EGF domain, a membrane binding domain (MBD) and a large C-terminal globular catalytic domain containing the heme binding site. The C-terminal segments beyond Pro583 (35 amino acids in COX-2) have not been resolved crystallographically. Collectively, these domains are made up of 25 <scene name='SandboxUAM/Mynewscene/23'>alpha helices</scene>, seven 3<sub>10</sub> <scene name='SandboxUAM/Mynewscene/20'>helices</scene>, four <scene name='SandboxUAM/Mynewscene/24'>beta sheets</scene> as well as five disulfide bonds which contribute to the interface binding of the two individual monomers to complete the enzyme. | The COX monomer consists of <scene name='SandboxUAM/Mynewscene/12'>three structural domains</scene>: the N-terminal EGF domain, a membrane binding domain (MBD) and a large C-terminal globular catalytic domain containing the heme binding site. The C-terminal segments beyond Pro583 (35 amino acids in COX-2) have not been resolved crystallographically. Collectively, these domains are made up of 25 <scene name='SandboxUAM/Mynewscene/23'>alpha helices</scene>, seven 3<sub>10</sub> <scene name='SandboxUAM/Mynewscene/20'>helices</scene>, four <scene name='SandboxUAM/Mynewscene/24'>beta sheets</scene> as well as five disulfide bonds which contribute to the interface binding of the two individual monomers to complete the enzyme. | ||
| Line 79: | Line 77: | ||
==NSAIDs== | ==NSAIDs== | ||
| - | <Structure load='3ln1' size='300' frame='true' align='right' caption=' | + | <Structure load='3ln1' size='300' frame='true' align='right' caption='COX-2 binded to celecoxib' scene='Insert optional scene name here' /> |
Non-steroid anti-inflammatory drugs are a chemically heterogeneous group of compounds whose major function is the inhibition of cyclooxygenases (Table 1). Apart from their anti-inflammatory effect, they also present analgesic and antipyretic properties <ref name="Rang&Dale2008"/>. | Non-steroid anti-inflammatory drugs are a chemically heterogeneous group of compounds whose major function is the inhibition of cyclooxygenases (Table 1). Apart from their anti-inflammatory effect, they also present analgesic and antipyretic properties <ref name="Rang&Dale2008"/>. | ||
| - | Classical NSAIDs, as salicylate or phenoprofen, are mostly inhibitors of both isoenzymes, although each isoform is inhibited in a different level (Table 2). Chronic users of NSAIDs develop gastric ulcers or gastrointestinal complications, explained by the inhibition of COX-1. For this reason, selective inhibitors of COX-2, as <scene name='SandboxUAM/Mynewscene/33 | + | Classical NSAIDs, as salicylate or phenoprofen, are mostly inhibitors of both isoenzymes, although each isoform is inhibited in a different level (Table 2). Chronic users of NSAIDs develop gastric ulcers or gastrointestinal complications, explained by the inhibition of COX-1. For this reason, selective inhibitors of COX-2, as <scene name='SandboxUAM/Mynewscene/33'>celecoxib</scene>, valdecoxib and etoricoxib, have been developed <ref name="Ghosh2010"/> <ref name="Rang&Dale2008"/>. They don’t cause gastric pathology, but they has been proven to be responsible of nephrotoxicity in some patients. |
The majority of NSAIDs inhibit competitively the initial dioxygenation <ref name="Ghosh2010"/> <ref name="Rang&Dale2008"/>. In general, these drugs block COX-1 in a quicker manner, whereas COX-2 inhibition is a more time-dependant event, and usually irreversible <ref name="Ghosh2010"/> <ref name="Rang&Dale2008"/>. The new COX-2 inhibitors exhibit PGHS-2 selectivity because they inhibit this isoform by a time-dependent <ref name="Fitzgerald2001">PMID: 11390412</ref> <ref name="Smith2000"/>, pseudoirreversible mechanism, whereas they inhibit PGHS-1 by a rapid, competitive, and reversible mechanism <ref name="Smith2000"/>. | The majority of NSAIDs inhibit competitively the initial dioxygenation <ref name="Ghosh2010"/> <ref name="Rang&Dale2008"/>. In general, these drugs block COX-1 in a quicker manner, whereas COX-2 inhibition is a more time-dependant event, and usually irreversible <ref name="Ghosh2010"/> <ref name="Rang&Dale2008"/>. The new COX-2 inhibitors exhibit PGHS-2 selectivity because they inhibit this isoform by a time-dependent <ref name="Fitzgerald2001">PMID: 11390412</ref> <ref name="Smith2000"/>, pseudoirreversible mechanism, whereas they inhibit PGHS-1 by a rapid, competitive, and reversible mechanism <ref name="Smith2000"/>. | ||
| Line 89: | Line 87: | ||
The inhibition mechanism consists of the entrance of the drug by the hydrophobic channel and the formation of hydrogen bonds with Arg120. This interaction prevents the fatty acids from entering the catalytic site. Selectivity of COX-2 inhibitors is mainly mediated by the substitution of Ile523 in COX-1 with Val523 in COX-2, which results in the presence of a small side pocket adjacent to the active site channel, appreciably increasing the volume of the COX-2 active site <ref name="Ghosh2010"/> <ref name="Rang&Dale2008"/> <ref name="Smith2000"/>. The effect of this change is compounded by the substitution of Val434 in COX-2 for Ile434 in COX-1 within the second group of amino acids conforming the active site <ref name="Garavito&DeWitt1999"/>. The combination of these two substitutions in COX-2 allows a neighboring amino acid, Phe518, to swing out of the way, which further increases access to the side pocket <ref name="Garavito&DeWitt1999"/>. | The inhibition mechanism consists of the entrance of the drug by the hydrophobic channel and the formation of hydrogen bonds with Arg120. This interaction prevents the fatty acids from entering the catalytic site. Selectivity of COX-2 inhibitors is mainly mediated by the substitution of Ile523 in COX-1 with Val523 in COX-2, which results in the presence of a small side pocket adjacent to the active site channel, appreciably increasing the volume of the COX-2 active site <ref name="Ghosh2010"/> <ref name="Rang&Dale2008"/> <ref name="Smith2000"/>. The effect of this change is compounded by the substitution of Val434 in COX-2 for Ile434 in COX-1 within the second group of amino acids conforming the active site <ref name="Garavito&DeWitt1999"/>. The combination of these two substitutions in COX-2 allows a neighboring amino acid, Phe518, to swing out of the way, which further increases access to the side pocket <ref name="Garavito&DeWitt1999"/>. | ||
| - | {| class="wikitable" align=left" | + | |
| + | |||
| + | {| class="wikitable" align="left" | ||
! scope="col" | Pharmacologic group | ! scope="col" | Pharmacologic group | ||
! scope="col" | Drug | ! scope="col" | Drug | ||
| Line 118: | Line 118: | ||
|} | |} | ||
| - | + | {| class="wikitable" align="right" | |
| - | {| class="wikitable" align=right" | + | |
|- | |- | ||
! Drug | ! Drug | ||
| Line 149: | Line 148: | ||
|+ Table 2: Selectivity of some NSAIDs (adapted from <ref name="Rang&Dale2008"/>) | |+ Table 2: Selectivity of some NSAIDs (adapted from <ref name="Rang&Dale2008"/>) | ||
|} | |} | ||
| - | + | ||
In addition, other NSAIDs present alternative inhibition mechanisms. Acetylsalicylic acid, for example, makes its function by irreversible acetylation of COX-2 in Ser516 <ref name="Rang&Dale2008"/>. | In addition, other NSAIDs present alternative inhibition mechanisms. Acetylsalicylic acid, for example, makes its function by irreversible acetylation of COX-2 in Ser516 <ref name="Rang&Dale2008"/>. | ||
Finally, paracetamol is considered an atypical NSAIDs, not only because of its lack of anti-inflammatory properties but also because it does not interact neither with COX-1 nor with COX-2 <ref name="Rang&Dale2008"/>. It has been proposed that paracetamol may act as an analgesic and antipyretic drug by inhibition of COX-3 <ref name="Rang&Dale2008"/>. | Finally, paracetamol is considered an atypical NSAIDs, not only because of its lack of anti-inflammatory properties but also because it does not interact neither with COX-1 nor with COX-2 <ref name="Rang&Dale2008"/>. It has been proposed that paracetamol may act as an analgesic and antipyretic drug by inhibition of COX-3 <ref name="Rang&Dale2008"/>. | ||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
Revision as of 21:53, 9 December 2010
COX-1 and COX-2, also called PGHS-1 and PGHS-2, regulate a key step in prostaglandin and thromboxane synthesis and are the targets of nonsteroidal antiinflammatory drugs (NSAIDs) [1] [2] [3]. Prostaglandins are implicated in various pathophysiological processes such as inflammatory reactions, gastrointestinal cytoprotection, hemostasis and thrombosis, as well as renal hemodynamics [1] [3] [4]. Whereas COX-1 presents a widespread constitutive expression, COX-2 is undetectable in most normal tissues (except for the central nervous system, kidneys, and seminal vesicles), but is induced by various inflammatory and mitogenic stimuli [4] [3] [5]. More recently, a third isoform named COX-3 was identified as a COX-1 splicing variant. This new isoform may play a role in processes such as fever and pain [3] [2]. Additionally, a high level of COX-2 expression is found usually in cancer cells [3]. For example, COX-2 overexpression is related to poor prognosis in certain breast cancers [6] [7] and endometrial adenocarcinomas [8].
Contents |
Function
COX-2, unlike COX-1, is induced in inflammatory cells when they are activated by various inflammatory and mitogenic stimuli [5] in order to produce the prostanoid mediators of the inflammation. Constitutive levels of COX-2 are generally low in most tissues, although there are some significant exceptions. For example, there is a considerable pool of “constitutive” COX-2 present in the central nervous system (CNS) and some other tissues, although its function is not yet completely clear [3].
Moreover, COX-1, that is present ubiquitously, has a “housekeeping” role in the body, being involved in tissue homeostasis, and it appears to be responsible for the production of the prostaglandins involved in gastric cytoprotection, platelet aggregation, renal blood flow autoregulation and the initiation of parturition [3].
Structure [9] [4]
| |||||||||||
Physiological Regulation
COX-2 overexpression is a very important process since it has significant tissue-specific consequences and is associated with inflammatory diseases, cancers and term/preterm labour, thus making COX-2 an important target for pharmacological intervention [16].
The expression of COX-2 in many specialized cell types appears to be differentially sensitive to the different stimuli that regulate the unique physiological activities of each tissue [4].
This physiological regulation can be produced at various levels [5]:
- Transcriptional regulation
- Post-transcriptional regulation: via 3’UTR, miRNAs (microRNAs) and alternative polyadenylation
Transcriptional regulation [5]
Transcriptional activation of COX-2 occurs quickly and transiently in response to different stimuli, for example: pathogens, cytokines, nitric oxide, irradiation, growth factors and various extracellular ligands.
The 5-UTR (untranslated region) of the COX-2 gene has several transcription factor response elements, including two NF-κB (nuclear factor κB) motifs, two AP-1 (activator protein 1) sites and two CREs (cAMP-response elements), among others [3]. Transcriptional regulation of COX-2 may also be physically influenced by chromatin remodelling events such as changes in acetylation status of histones and non-histone proteins. For example, the acetylation of NF-κB components by the transcriptional coativator p300 (histone acetyltransferase [HAT]) can activate the COX-2 expression [17], while the hypermethylation of the CpG islands results in transcriptional silencing [18]. It is also known that the histone deacetylase inhibitors (iHDAC) suppress the activation of the expression in human primary myometrial cells [19] and in cancer cell lines [20], by preventing the binding of the transcription factor, c-Jun, to the COX-2 promoter [20].
Post-transcriptional regulation [5]
Via 3’-UTR
The 3’-UTR of COX-2 is a complex region that contains multiple copies of AREs (AU-rich elements) throughout sequence, which, when bound by specific trans-acting ARE-binding factors, influence COX-2 mRNA stability and also translational efficiency [21]. A lot of studies have introduced a new model to the gene regulation of COX-2 by investigating the combined contribution of both transcription and mRNA stability events. For example, one group has reported that the binding of the protein CUGBP2 (CUG triplet repeat, RNA-binding protein 2) in specific AREs within the first 60 nucleotides of the COX-2 3’-UTR can stabilize the COX-2 mRNA inhibiting its translation [22]. Also, there is evidence that mitogenic inhibitors (e.g. taxanes) can control COX-2 transcription via PKC (Protein Kinase C)-p38 MAPK (Mitogen-Activated Protein Kinase) signaling cascade and it is known that the stability of COX-2 mRNA can be controlled by the binding of HuR (a mRNA-stabilizing factor) to AREs in 3’-UTR of COX-2.
miRNAs (microRNAs)
A recent study has demonstrated that the microRNAs miR-101a and miRNA-199a can interact with the COX-2 3’-UTR in vitro thus repressing its translation [23].
Alternative polyadenylation
The human COX-2 3’-UTR has several polyadenylation sites. COX-2 uses two alternative polyadenylation sites, in a tissue-specific manner, which derives in the formation of 2 COX-2 mRNAs: one with 2.8 kb and another one with 4.6 kb [24]. It is known that selection of the proximal polyadenylation signal is enhanced by presence of additional USEs (Upstream Sequence Elements) where four RNA-binding proteins (U1A, PTB, p54nrb and PSF) can bind, enhancing the recruitment and stabilization of core adenylation factors on the COX-2 mRNA [25].
NSAIDs
|
Non-steroid anti-inflammatory drugs are a chemically heterogeneous group of compounds whose major function is the inhibition of cyclooxygenases (Table 1). Apart from their anti-inflammatory effect, they also present analgesic and antipyretic properties [5].
Classical NSAIDs, as salicylate or phenoprofen, are mostly inhibitors of both isoenzymes, although each isoform is inhibited in a different level (Table 2). Chronic users of NSAIDs develop gastric ulcers or gastrointestinal complications, explained by the inhibition of COX-1. For this reason, selective inhibitors of COX-2, as , valdecoxib and etoricoxib, have been developed [3] [5]. They don’t cause gastric pathology, but they has been proven to be responsible of nephrotoxicity in some patients.
The majority of NSAIDs inhibit competitively the initial dioxygenation [3] [5]. In general, these drugs block COX-1 in a quicker manner, whereas COX-2 inhibition is a more time-dependant event, and usually irreversible [3] [5]. The new COX-2 inhibitors exhibit PGHS-2 selectivity because they inhibit this isoform by a time-dependent [26] [4], pseudoirreversible mechanism, whereas they inhibit PGHS-1 by a rapid, competitive, and reversible mechanism [4].
The inhibition mechanism consists of the entrance of the drug by the hydrophobic channel and the formation of hydrogen bonds with Arg120. This interaction prevents the fatty acids from entering the catalytic site. Selectivity of COX-2 inhibitors is mainly mediated by the substitution of Ile523 in COX-1 with Val523 in COX-2, which results in the presence of a small side pocket adjacent to the active site channel, appreciably increasing the volume of the COX-2 active site [3] [5] [4]. The effect of this change is compounded by the substitution of Val434 in COX-2 for Ile434 in COX-1 within the second group of amino acids conforming the active site [15]. The combination of these two substitutions in COX-2 allows a neighboring amino acid, Phe518, to swing out of the way, which further increases access to the side pocket [15].
| Pharmacologic group | Drug |
|---|---|
| Salicylates | Acetylsalicylic acid |
| Propionic | Naproxen |
| Ibuprofen | |
| Para-aminophenols | Paracetamol |
| Indolacetic | Indometacin |
| Pirrolacetic | Ketorolac |
| Phenilacetic | Diclofenac |
| Piranoidacetic | Etodolac |
| Anthranilic | Mefenamic acid |
| Nicotinic | Clonixin |
| Sulfonanilides | Nimesulide |
| Drug | Coefficient of selectivity (IC50Cox-1/IC50Cox-2) |
|---|---|
| Ketorolac | |
| Naproxen | |
| Ibuprofen | |
| Indometacin | |
| Acetylsalicylic acid | |
| Diclofenac | |
| Valdecoxib | |
| Etoricoxib | |
In addition, other NSAIDs present alternative inhibition mechanisms. Acetylsalicylic acid, for example, makes its function by irreversible acetylation of COX-2 in Ser516 [5].
Finally, paracetamol is considered an atypical NSAIDs, not only because of its lack of anti-inflammatory properties but also because it does not interact neither with COX-1 nor with COX-2 [5]. It has been proposed that paracetamol may act as an analgesic and antipyretic drug by inhibition of COX-3 [5].
Reference
- ↑ 1.0 1.1 Smith WL, Langenbach R. Why there are two cyclooxygenase isozymes. J Clin Invest. 2001 Jun;107(12):1491-5. PMID:11413152 doi:10.1172/JCI13271
- ↑ 2.0 2.1 Chandrasekharan NV, Dai H, Roos KL, Evanson NK, Tomsik J, Elton TS, Simmons DL. COX-3, a cyclooxygenase-1 variant inhibited by acetaminophen and other analgesic/antipyretic drugs: cloning, structure, and expression. Proc Natl Acad Sci U S A. 2002 Oct 15;99(21):13926-31. Epub 2002 Sep 19. PMID:12242329 doi:10.1073/pnas.162468699
- ↑ 3.00 3.01 3.02 3.03 3.04 3.05 3.06 3.07 3.08 3.09 3.10 3.11 Ghosh N, Chaki R, Mandal V, Mandal SC. COX-2 as a target for cancer chemotherapy. Pharmacol Rep. 2010 Mar-Apr;62(2):233-44. PMID:20508278
- ↑ 4.0 4.1 4.2 4.3 4.4 4.5 4.6 Smith WL, DeWitt DL, Garavito RM. Cyclooxygenases: structural, cellular, and molecular biology. Annu Rev Biochem. 2000;69:145-82. PMID:10966456 doi:10.1146/annurev.biochem.69.1.145
- ↑ 5.00 5.01 5.02 5.03 5.04 5.05 5.06 5.07 5.08 5.09 5.10 5.11 5.12 5.13 Rang HP, Dale MM, Ritter JM, Flower RJ. 2008. Pharmacology. Elsevier. 6th edition. UK. 844 p.
- ↑ Barnes NL, Warnberg F, Farnie G, White D, Jiang W, Anderson E, Bundred NJ. Cyclooxygenase-2 inhibition: effects on tumour growth, cell cycling and lymphangiogenesis in a xenograft model of breast cancer. Br J Cancer. 2007 Feb 26;96(4):575-82. Epub 2007 Feb 6. PMID:17285134 doi:10.1038/sj.bjc.6603593
- ↑ Boland GP, Butt IS, Prasad R, Knox WF, Bundred NJ. COX-2 expression is associated with an aggressive phenotype in ductal carcinoma in situ. Br J Cancer. 2004 Jan 26;90(2):423-9. PMID:14735188 doi:10.1038/sj.bjc.6601534
- ↑ Sales KJ, Grant V, Jabbour HN. Prostaglandin E2 and F2alpha activate the FP receptor and up-regulate cyclooxygenase-2 expression via the cyclic AMP response element. Mol Cell Endocrinol. 2008 Mar 26;285(1-2):51-61. Epub 2008 Feb 3. PMID:18316157 doi:10.1016/j.mce.2008.01.016
- ↑ Perrone G, Zagami M, Altomare V, Battista C, Morini S, Rabitti C. COX-2 localization within plasma membrane caveolae-like structures in human lobular intraepithelial neoplasia of the breast. Virchows Arch. 2007 Dec;451(6):1039-45. Epub 2007 Sep 13. PMID:17851687 doi:10.1007/s00428-007-0506-4
- ↑ Luong C, Miller A, Barnett J, Chow J, Ramesha C, Browner MF. Flexibility of the NSAID binding site in the structure of human cyclooxygenase-2. Nat Struct Biol. 1996 Nov;3(11):927-33. PMID:8901870
- ↑ Kurumbail RG, Stevens AM, Gierse JK, McDonald JJ, Stegeman RA, Pak JY, Gildehaus D, Miyashiro JM, Penning TD, Seibert K, Isakson PC, Stallings WC. Structural basis for selective inhibition of cyclooxygenase-2 by anti-inflammatory agents. Nature. 1996 Dec 19-26;384(6610):644-8. PMID:8967954 doi:http://dx.doi.org/10.1038/384644a0
- ↑ Spencer AG, Thuresson E, Otto JC, Song I, Smith T, DeWitt DL, Garavito RM, Smith WL. The membrane binding domains of prostaglandin endoperoxide H synthases 1 and 2. Peptide mapping and mutational analysis. J Biol Chem. 1999 Nov 12;274(46):32936-42. PMID:10551860
- ↑ Vecchio AJ, Simmons DM, Malkowski MG. Structural basis of fatty acid substrate binding to cyclooxygenase-2. J Biol Chem. 2010 Jul 16;285(29):22152-63. Epub 2010 May 12. PMID:20463020 doi:10.1074/jbc.M110.119867
- ↑ Luong C, Miller A, Barnett J, Chow J, Ramesha C, Browner MF. Flexibility of the NSAID binding site in the structure of human cyclooxygenase-2. Nat Struct Biol. 1996 Nov;3(11):927-33. PMID:8901870
- ↑ 15.0 15.1 15.2 Garavito RM, DeWitt DL. The cyclooxygenase isoforms: structural insights into the conversion of arachidonic acid to prostaglandins. Biochim Biophys Acta. 1999 Nov 23;1441(2-3):278-87. PMID:10570255
- ↑ Harper KA, Tyson-Capper AJ. Complexity of COX-2 gene regulation. Biochem Soc Trans. 2008 Jun;36(Pt 3):543-5. PMID:18482003 doi:10.1042/BST0360543
- ↑ Deng WG, Zhu Y, Wu KK. Up-regulation of p300 binding and p50 acetylation in tumor necrosis factor-alpha-induced cyclooxygenase-2 promoter activation. J Biol Chem. 2003 Feb 14;278(7):4770-7. Epub 2002 Dec 5. PMID:12471036 doi:10.1074/jbc.M209286200
- ↑ Song SH, Jong HS, Choi HH, Inoue H, Tanabe T, Kim NK, Bang YJ. Transcriptional silencing of Cyclooxygenase-2 by hyper-methylation of the 5' CpG island in human gastric carcinoma cells. Cancer Res. 2001 Jun 1;61(11):4628-35. PMID:11389100
- ↑ Tyson-Capper AJ, Cork DM, Wesley E, Shiells EA, Loughney AD. Characterization of cellular retinoid-binding proteins in human myometrium during pregnancy. Mol Hum Reprod. 2006 Nov;12(11):695-701. Epub 2006 Sep 7. PMID:16959971 doi:10.1093/molehr/gal070
- ↑ 20.0 20.1 Yamaguchi K, Lantowski A, Dannenberg AJ, Subbaramaiah K. Histone deacetylase inhibitors suppress the induction of c-Jun and its target genes including COX-2. J Biol Chem. 2005 Sep 23;280(38):32569-77. Epub 2005 Jul 1. PMID:15994313 doi:10.1074/jbc.M503201200
- ↑ Dixon DA, Kaplan CD, McIntyre TM, Zimmerman GA, Prescott SM. Post-transcriptional control of cyclooxygenase-2 gene expression. The role of the 3'-untranslated region. J Biol Chem. 2000 Apr 21;275(16):11750-7. PMID:10766797
- ↑ Mukhopadhyay D, Houchen CW, Kennedy S, Dieckgraefe BK, Anant S. Coupled mRNA stabilization and translational silencing of cyclooxygenase-2 by a novel RNA binding protein, CUGBP2. Mol Cell. 2003 Jan;11(1):113-26. PMID:12535526
- ↑ Subbaramaiah K, Marmo TP, Dixon DA, Dannenberg AJ. Regulation of cyclooxgenase-2 mRNA stability by taxanes: evidence for involvement of p38, MAPKAPK-2, and HuR. J Biol Chem. 2003 Sep 26;278(39):37637-47. Epub 2003 Jun 25. PMID:12826679 doi:10.1074/jbc.M301481200
- ↑ Hall-Pogar T, Zhang H, Tian B, Lutz CS. Alternative polyadenylation of cyclooxygenase-2. Nucleic Acids Res. 2005 May 4;33(8):2565-79. Print 2005. PMID:15872218 doi:10.1093/nar/gki544
- ↑ Hall-Pogar T, Liang S, Hague LK, Lutz CS. Specific trans-acting proteins interact with auxiliary RNA polyadenylation elements in the COX-2 3'-UTR. RNA. 2007 Jul;13(7):1103-15. Epub 2007 May 16. PMID:17507659 doi:10.1261/rna.577707
- ↑ FitzGerald GA, Loll P. COX in a crystal ball: current status and future promise of prostaglandin research. J Clin Invest. 2001 Jun;107(11):1335-7. PMID:11390412 doi:10.1172/JCI13037
Proteopedia Page Contributors and Editors (what is this?)
Cristina Murga, Michal Harel, David Canner, María Laura Saiz Álvarez, Alexander Berchansky