Aminopeptidase
From Proteopedia
| Line 3: | Line 3: | ||
'''Aminopeptidases''' catalyze a release of an N-terminal amino acid from a peptide, amide, or arylamide. | '''Aminopeptidases''' catalyze a release of an N-terminal amino acid from a peptide, amide, or arylamide. | ||
| - | + | {{Clear}} | |
== ''S. griseus'' aminopeptidase == | == ''S. griseus'' aminopeptidase == | ||
Revision as of 11:34, 30 December 2010
Aminopeptidases catalyze a release of an N-terminal amino acid from a peptide, amide, or arylamide.
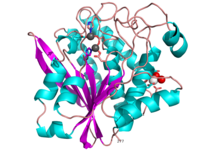
S. griseus aminopeptidase
|
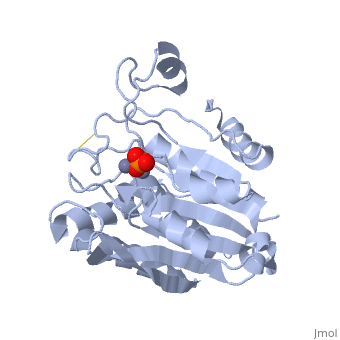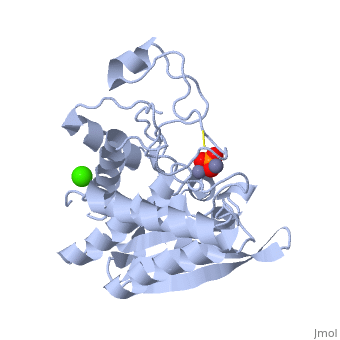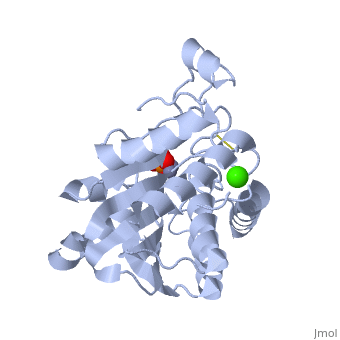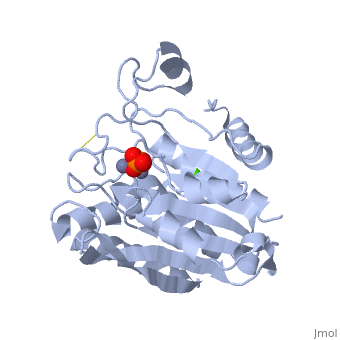
S. griseus aminopeptidase (SGAP) cleaves the N-terminal amino acid from a peptide or protein, and is specific for larger hydrophobic acids, especially leucine. No cleavage occurs if the next residue is proline.
The of the enzyme contains two Zn2+ ions with His85 and Asp160 as ligands for one ion, and Glu132 and His247 as ligands for the second ion. Asp97 is a common ligand to both ions. What appears to be a phosphate anion is bound to both zinc atoms, replacing the water molecule/hydroxide ion normally found in this class of enzyme.
Additional Resources
For additional information, see: Amino Acid Synthesis & Metabolism
Proteopedia Page Contributors and Editors (what is this?)
Michal Harel, Alexander Berchansky, David Canner, Joel L. Sussman, Eran Hodis