Citrate Synthase
From Proteopedia
m |
m |
||
| Line 10: | Line 10: | ||
{{Link Toggle FancyCartoonHighQualityView}} | {{Link Toggle FancyCartoonHighQualityView}} | ||
| - | <StructureSection load='Image:5ctsBIOL.pdb.gz' size=' | + | <StructureSection load='Image:5ctsBIOL.pdb.gz' size='380' side='right' scene='Citrate_Synthase/Activesitedimer/2' caption='Citrate synthase catalysis'> |
| - | '''Mechanism:''' <scene name='Citrate_Synthase/Activesitedimer/ | + | '''Mechanism:''' <scene name='Citrate_Synthase/Activesitedimer/2'>Three sidechains in each of the two active sites</scene> of the dimer contribute directly to the chemistry of catalysis. The reaction mechanism for citrate synthase was proposed by Remington and colleagues<ref name="1cts">PMID:7120407</ref><ref>PMID: 2337600</ref>. In this mechanism, three ionizable side chains in the |
<scene name='Daniel_Eddelman_Sandbox_2/Cts_active_site/4' >active site</scene> of citrate synthase participate in acid-base catalysis: His 274, His 320, and Asp 375. First, <scene name='Daniel_Eddelman_Sandbox_2/Asp_375/1'>Asp 375</scene> (a base) removes a proton from the methyl group of acetyl-CoA to form its enol. <scene name='Daniel_Eddelman_Sandbox_2/His_274/1'>His 274</scene> stabilizes the acetyl-CoA enolate by forming a hydrogen bond with the enolate oxygen. The enolate then nucleophilically attacks oxaloacetate’s carbonyl carbon, and | <scene name='Daniel_Eddelman_Sandbox_2/Cts_active_site/4' >active site</scene> of citrate synthase participate in acid-base catalysis: His 274, His 320, and Asp 375. First, <scene name='Daniel_Eddelman_Sandbox_2/Asp_375/1'>Asp 375</scene> (a base) removes a proton from the methyl group of acetyl-CoA to form its enol. <scene name='Daniel_Eddelman_Sandbox_2/His_274/1'>His 274</scene> stabilizes the acetyl-CoA enolate by forming a hydrogen bond with the enolate oxygen. The enolate then nucleophilically attacks oxaloacetate’s carbonyl carbon, and | ||
<scene name='Daniel_Eddelman_Sandbox_2/His_320/1'>His 320</scene> donates a proton to oxaloacetate’s carbonyl group in a concerted step, forming citryl-CoA (which remains bound to the enzyme). Finally, citryl-CoA is hydrolyzed to citrate and CoA. | <scene name='Daniel_Eddelman_Sandbox_2/His_320/1'>His 320</scene> donates a proton to oxaloacetate’s carbonyl group in a concerted step, forming citryl-CoA (which remains bound to the enzyme). Finally, citryl-CoA is hydrolyzed to citrate and CoA. | ||
Revision as of 21:19, 20 February 2011
Contents |
The Structure and Mechanism of Citrate Synthase
|
Citrate synthase is an enzyme active in the mitochondria, where it is responsible for catalyzing the first reaction of the citric acid cycle (Krebs Cycle): the condensation of acetyl-CoA and oxaloacetate to form citrate. Although it is a mitochondrial enzyme, and in fact, is often used as a enzyme marker for intact mitochondria, it is encoded by nuclear DNA, not mitochondrial [1]. The standard free energy change (ΔG°’) for the citrate synthase reaction is -31.5kJ/mol [2]. This negative free energy value means that citrate synthase is likely to function far from equilibrium under physiological conditions, and is thus a rate-determining enzyme in the citric acid cycle.
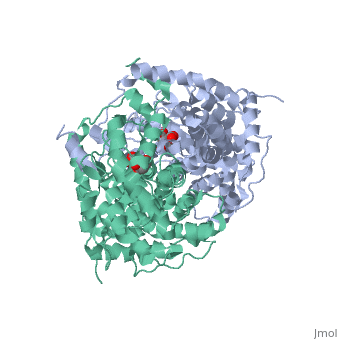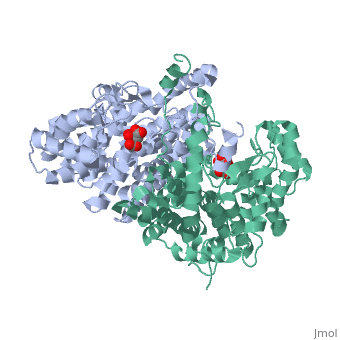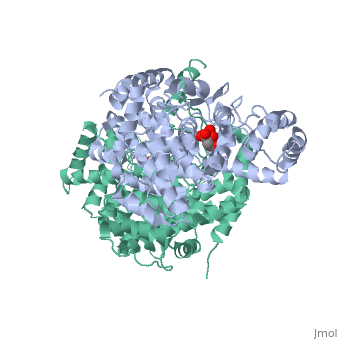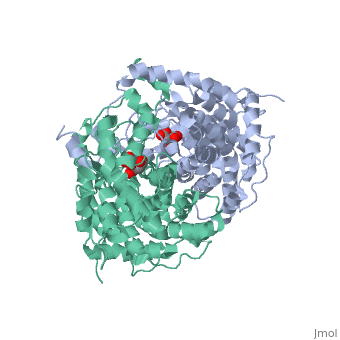
Structure: Biologically, citrate synthase exists as a
of a single amino acid chain . Each identical subunit consists of a large and a small domain, and is comprised almost entirely of α helices (making it an all α protein). In its free enzyme state, citrate synthase exists in , with its two domains forming a cleft containing the substrate (oxaloacetate) binding site (PDB: 1cts) [3][4]. When oxaloacetate binds, the smaller domain undergoes an 18° rotation, sealing the oxaloacetate binding site[5] and resulting in the (PDB: 2cts)[3]. The dramatic conformational change is best illustrated via , and be sure to view as well to get a full sense of the structural change. The conformational change not only prevents solvent from reaching the bound substrate, but also generates the acetyl-CoA binding site. This presence of “open” and “closed” forms results in citrate synthase having Ordered Sequential kinetic behavior [2].
| |||||||||||
|
|
Literature and Notes
- ↑ "Citrate Synthase -." Wikipedia, the Free Encyclopedia. Web. 22 Mar. 2010.
- ↑ 2.0 2.1 Voet, Donald, Judith G. Voet, and Charlotte W. Pratt. Fundamentals of Biochemistry: Life at the Molecular Level. Hoboken, NJ: Wiley, 2008.
- ↑ 3.0 3.1 3.2 Remington S, Wiegand G, Huber R. Crystallographic refinement and atomic models of two different forms of citrate synthase at 2.7 and 1.7 A resolution. J Mol Biol. 1982 Jun 15;158(1):111-52. PMID:7120407
- ↑ In this structure 1cts, citrate, the resulting product of the conversion, is actually bound where oxaloacetate binds.
- ↑ Bayer E, Bauer B, Eggerer H. Evidence from inhibitor studies for conformational changes of citrate synthase. Eur J Biochem. 1981 Nov;120(1):155-60. PMID:7308213
- ↑ Karpusas M, Branchaud B, Remington SJ. Proposed mechanism for the condensation reaction of citrate synthase: 1.9-A structure of the ternary complex with oxaloacetate and carboxymethyl coenzyme A. Biochemistry. 1990 Mar 6;29(9):2213-9. PMID:2337600
- ↑ Wiegand G, Remington SJ. Citrate synthase: structure, control, and mechanism. Annu Rev Biophys Biophys Chem. 1986;15:97-117. PMID:3013232 doi:http://dx.doi.org/10.1146/annurev.bb.15.060186.000525
Details of Structures Featured
1cts is a 1 chain structure with sequence from Sus scrofa. Full crystallographic information is available from OCA.
2cts is a 1 chain structure of sequence from Sus scrofa. Full crystallographic information is available from OCA.
3cts is a 1 chain structure of sequence from Gallus gallus. Full crystallographic information is available from OCA.
5cts is a 1 chain structure of sequence from Gallus gallus. Full crystallographic information is available from OCA.
6cts is a 1 chain structure of sequence from Gallus gallus. Full crystallographic information is available from OCA.
See Also
External Resources
Citrate Synthase: September 2007 Molecule of the Month as part of the series of tutorials that are at the RCSB Protein Data Bank and written by David Goodsell
Proteopedia Page Contributors and Editors (what is this?)
Wayne Decatur, Michal Harel, Daniel Eddelman, Alexander Berchansky, Joel L. Sussman, Angel Herraez, David Canner, Eric Martz