This old version of Proteopedia is provided for student assignments while the new version is undergoing repairs. Content and edits done in this old version of Proteopedia after March 1, 2026 will eventually be lost when it is retired in about June of 2026.
Apply for new accounts at the new Proteopedia. Your logins will work in both the old and new versions.
Sandbox 51
From Proteopedia
| Line 93: | Line 93: | ||
As seen above, lysozyme works by hydrolyzing the glycosidic bond, distorting the bond between the N-acetylglucosamine sugar and the N-acetylmuramic acid sugar. This produces a glycosyl enzyme intermediate, which reacts with a water molecule to produce the product and the unchanged enzyme. | As seen above, lysozyme works by hydrolyzing the glycosidic bond, distorting the bond between the N-acetylglucosamine sugar and the N-acetylmuramic acid sugar. This produces a glycosyl enzyme intermediate, which reacts with a water molecule to produce the product and the unchanged enzyme. | ||
| + | |||
| + | === Inhibitors === | ||
| + | |||
| + | Lysozyme is best inhibited by small saccharides which act competitively with the natural substrate. The smaller saccharides will bind to the first three binding sites of the cleft (sites A-C), but not reach sites D and E, where the enzyme cuts the glycosidic bond. So, the competitive inhibitor will stick in the cleft, not allowing the substrate to bind to the enzyme complex.<ref>http://mcdb-webarchive.mcdb.ucsb.edu/sears/biochemistry/tw-enz/lysozyme/HEWL/lysozyme-overview.htm</ref> Several known inhibitors of lysozyme are SDS, N-acetyl-D-glucosamine, and various alcohols and oxidizing agents.<ref>http://www.worthington-biochem.com/ly/default.html</ref> | ||
| + | |||
=== Zymogen of Lysozyme: Enzymatic Precursor === | === Zymogen of Lysozyme: Enzymatic Precursor === | ||
Revision as of 21:19, 5 March 2011
| Please do NOT make changes to this Sandbox. Sandboxes 30-60 are reserved for use by Biochemistry 410 & 412 at Messiah College taught by Dr. Hannah Tims during Fall 2012 and Spring 2013. |
Contents |
Lysozyme
Introduction
Lysozyme is a powerful enzyme of biological significance found in abundance in tears, saliva, and human milk. In humans, it is encoded in the LYZ gene. Lysozyme is also known as muramidase, or glycocide hydrolase. Since it is a small, easily available, and highly stable protein containing only 129 amino acid residues, it has been subject to extensive research regarding its function and structure.
A simple cartoon structure of lysozyme can be seen below in the Jmol box to the right. The secondary structures can be seen in blue, and the disulfide bonds are highlighted in yellow. The following sections will highlight different subsections of the lysozyme protein using colors and labels through the program Jmol. The PDB ID used to represent lysozyme throughout this page is 1HEW.
Function
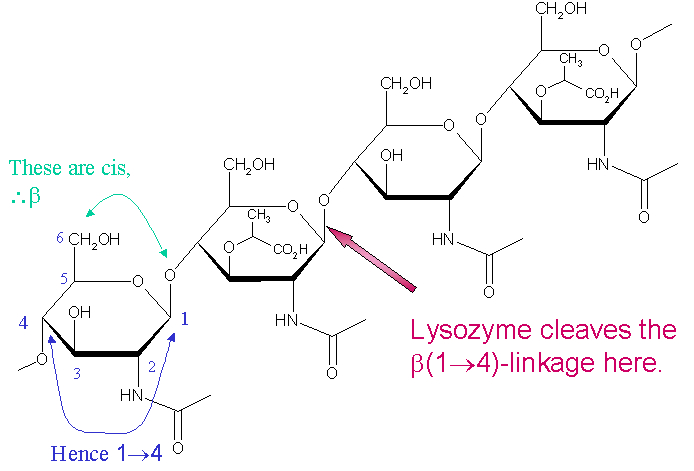
Lysozyme is known for damaging bacterial cell walls by catalyzing the hydrolysis of 1,4-beta-linkages between N-acetylmuramic acid and N-acetyl-D-glucosamine residues in a peptidoglycan and between N-acetyl-D-glucosamine residues in chitodextrins. In this way, lysozyme is efficient in lysing the cell walls of both bacteria and fungi. The location of cleavage for lysozyme on this architectural theme is the β(1-4) glycosidic linkage connecting connecting the C1 carbon of NAM to the C4 carbon of NAG.
Although it is responsible for the initial digestion of starches in the mouth, it is most widely used as a non-specific defense to gram positive bacteria and many species of fungi. Due to its antibacterial effects, it is a strong component of the innate immune system, and is an important part of an infant's diet to ward off diarrheal diseases.
History
Lysozyme is an enzyme known for its unique ability to degrade the polysaccharide architecture of many kinds of cell walls, normally for the purpose of protection against bacterial infection[2]. Its properties were first noticed by Laschtschenko in 1909, and then was officially named lysozyme by Alexander Fleming, the same person credited for the accidental discovery of penicillin. The identification of lysozyme in 1922 by Alexander Fleming was providential in that the undertaken experiment related to the discovery of lysozyme was not geared toward any knowledge of such a protein as lysozyme [3]. During the unrelated experiment, nasal drippings were inadvertently introduced to a petri dish containing a bacterial culture, which culture consequently exhibited the results of an as yet unknown enzymatic reaction. The observation of this unknown reaction led to further research on the components of this reaction as well as to the corresponding identification of the newfound "lysozyme." Fleming's discovery was complemented by David C. Phillips' 1965 description of the three-dimensional structure of lysozyme via a 200pm resolution model obtained from X-ray crystallography [4]. Phillips' work was especially groundbreaking since, by successfully elucidating the structure of lysozyme via X-ray crystallography, Phillips had managed to successfully elucidate the structure of an enzyme via X-ray crystallography - a feat that had never before been accomplished[5]. Phillips' research also led to the first sufficiently described enzymatic mechanism of catalytic action [6]. Thus, Phillips' elucidation of the function of lysozyme led Phillips to reach a more general conclusion on the diversity of enzymatic chemical action in relation to enzymatic structure. Clearly, the findings of Phillips as well as the more general historical development of the understanding of the structure and function of lysozyme have been paramount to the more general realm of enzyme chemistry.
|
Composition and Structure of Lysozyme
All proteins consist of carbon, hydrogen, nitrogen, oxygen, and sulfur, as do most organic molecules. Enzymes are composed in such a way as to maximize their reactivity with their desired substrate, increasing the efficiency of biological reactions. The can be seen on the left, with the carbon atoms outlined in gray, oxygen atoms in red, nitrogen atoms in blue, sulfur atoms in yellow, and the three-letter abbreviation for the in purple.
Lysozyme, like all proteins, also contains a , and these can be seen by following the colors of the rainbow across the molecule. Starting at the red end, the 3' C terminal end, one can work the entire way through to the 5' N terminal end, showing the folding pattern and chain of the protein.
Disulfide Bonding in Lysozyme
Lysozyme contains four involving eight cystine residues, which are highlighted in yellow on the left. Disulfide bonds are intramolecular forces that stabalize the tertiary structure of many proteins, and in this case, Lysozyme. Disulfide bonds are present in four locations in lysozyme: between Cys 6 and Cys 127, between Cys 30 and Cys 115, between Cys 64 and Cys 80 and between Cys 76 and Cys 94.
Secondary Structure
The structure of lysozyme with its highlighted in yellow and pink can be seen to your left. The structures highlight the alpha helicies, and the yellow lines highlight the beta-pleated sheets. This depiction of Lysozyme contains six alpha helicies and three beta-pleated sheets, although the number of alpha helicies and beta pleated sheet in lysozyme can change depending on its structure in its original location. This depiction of lysozyme contains an antiparallel beta-pleated sheet, which contributes greatly to the stability of the molecule by providing the correct alignment of hydrogen bonds. Lysozyme also contains a great deal of random coil, which is seen in the white regions of the molecule.
|
Hydrophobicity
Lysozyme contain both hydrophobic, or water-hating, regions and hydrophillic, or water-loving, regions, referred to overall as . The hydrophilic effect, or the desire for proteins to be at a specific position regarding water, is the single most important determinant of protein folding. These regions can be displayed with the hydrophobic regions in gray and the polar, hydrophillic regions in purple. This coloration highlights the location of these regions, showing the majority of the hydrophobic regions are inside of the protein and the majority of the hydrophillic regions are on the outside of the molecule.
Here, lysozyme can also be seen interacting with , demonstrating how water remains almost exclusively on the outside of the molecule where the polar residues reside.
Charged Residues
The charged residues, or the most polar portions of the molecule, are seen highlighted to the right in the space-filling model. The blue represent cations, and the red charges represent anions. The can also be seen, with the charged residues the same as above and the polar residues in purple. It is important to note that these residues are found almost exclusively on the outside of the protein to increase its interaction of water.
Hydrogen Bonding
Like all proteins, are essential for the stability of a protein. In this ribbon diagram, the hydrogen bonds can be seen between the secondary structures of the protein highlighted in orange. Since the double bonds of the alpha carbons in the main chain of Lysozyme cause torsinal strain, lysozyme is limited to very specific hydrogen bonding between the amino acid residues. This representation clearly shows how crucial hydrogen bonding is to help maintain the stability of the protein.
|
Enzymatic Activity of Lysozyme
Enzymes are specifically designed to attract and bind the specific substrate they were designed to bind. The active site and lysozyme's specific ligands are discribed in the following sections
Active Site
The of lysozyme is formulated as a prominent cleft outlined hy the two aforementioned catalytic enzymes, Glu 35 and Asp 52. The active site is bent to geometrically attract the shape of the ligand it binds, and the two amino acids interact with the ligand in the binding site. Asp52 is depicted in green, and Glu35 is depicted in purple.
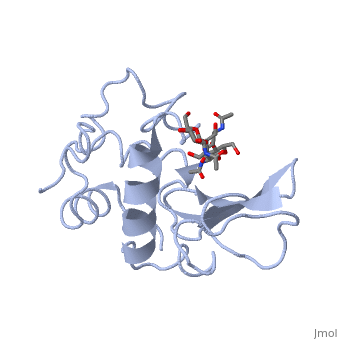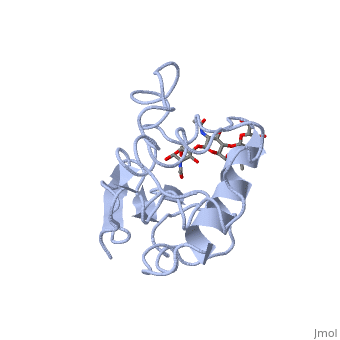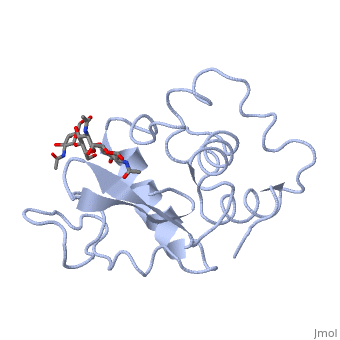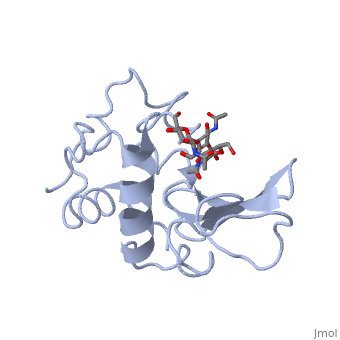
Ligands
A is a protein that is able to bind to the active site of an enzyme to form a biologically relevant complex. The model to your left shows a space-filling model of lysozyme, with the protein distinguishable in brown and the ligand distinguishable in green. Another model of the ligand can be seen in this , with the ligand protruding as a space-filling model from the active site. Here, it is clear that the ligand is composed of a polysaccharide.
Lysozyme reaction is hydrolysis of the beta (1-4) glycosidic bond between N-acetylglucosamine sugar (NAG) and N-acetylmuramic acid sugar (NAM). Lysozyme has a very specific active site, which can bind only six sugar rings from a polysaccharide chain. Once lysozyme binds to these sugars, it hydrolyzes them. It is these six sugar rings represent the ligand of lysozyme. The lysozyme then distorts the fourth sugar in the six membered sugar, producing stress on the molecule and breaking the glycosidic bond.
The amino acid side-chains glutamic acid 35 (Glu35) and aspartate 52 (Asp52) have been found to be critical to the activity of this enzyme. Glu35 acts as a proton donor to the glycosidic bond, cleaving the C-O bond in the substrate, whereas Asp52 acts as a nucleophile to generate a glycosyl enzyme intermediate. The glycosyl enzyme intermediate then reacts with a water molecule, to give the product of hydrolysis and leaving the enzyme unchanged.
Mechanism
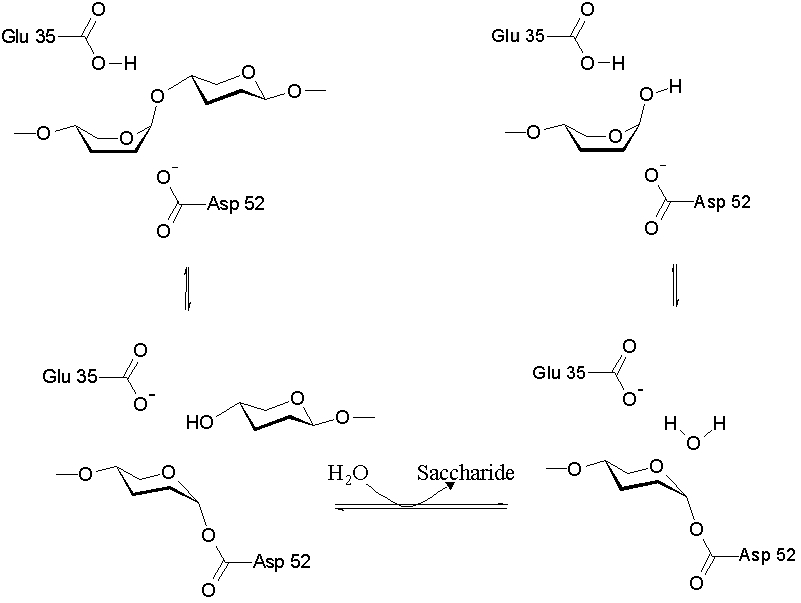
The lysozyme mechanism of action results in the hydrolysis of a glycoside (hence the familial distinction of lysozyme as a glycosylase[7]), which corresponds to the conversion of an acetal to a hemiacetal, which reaction (general degradation of glycosidic bond to units "capped" by newly formed hydroxyl groups) necessitates acid catalysis, since the conversion of acetal to hemiacetal involves the protonation of the reactant oxygen prior to actual bond cleavage. [8]. Furthermore, the transition state obtained from this protonation is a covalent, oxonium ion, intermediate that must obtain resonance stabilization. The need for some means of acid catalysis and covalent resonance stabilization is adequately provided by the Glu 35 and Asp 52 residues of lysozyme, respectively. The reaction mechanism of lysozyme is demonstrated below. In the following image, the reaction begins at the upper left-hand side, and proceeds according to reaction arrows.
 [9]
[9]
As seen above, lysozyme works by hydrolyzing the glycosidic bond, distorting the bond between the N-acetylglucosamine sugar and the N-acetylmuramic acid sugar. This produces a glycosyl enzyme intermediate, which reacts with a water molecule to produce the product and the unchanged enzyme.
Inhibitors
Lysozyme is best inhibited by small saccharides which act competitively with the natural substrate. The smaller saccharides will bind to the first three binding sites of the cleft (sites A-C), but not reach sites D and E, where the enzyme cuts the glycosidic bond. So, the competitive inhibitor will stick in the cleft, not allowing the substrate to bind to the enzyme complex.[10] Several known inhibitors of lysozyme are SDS, N-acetyl-D-glucosamine, and various alcohols and oxidizing agents.[11]
Zymogen of Lysozyme: Enzymatic Precursor
Zymogens are enzymatic precursors to active enzymes, which are developed in an inactive way to prevent the enzyme from digesting the cell that produced it, or becoming inactive in the wrong portion of the body. Lysozyme's zymogen, simply titled pre-lysozyme, was sequenced in 1977 by R D Palmiter, J Gagnon, L H Ericsson and K A Walsh, and since then it has been sequenced much more extensively.
Applications of Lysozyme
Since lysozyme has been widely recognized for its antibacterial and antifungal properties, it has a wide variety of uses both in biochemical and pharmaceutical applications. In molecular biology, lysozyme is often used in the alkaline-lysis procedure for extracting and isolating plasmid DNA. It is used extensively in the pharmaceutical field for destroying gram-positive bacteria, and can be used to support already-existing immune defenses to fight bacterial infections. This enzyme is particularly important for preventing bacterial disease in infants. Because of its antibacterial properties, lysozyme can also be used in the food industry to help prevent spoilage of foods.
References
- ↑ Image from: http://www.vuw.ac.nz/staff/paul_teesdale-spittle/essentials/chapter-6/proteins/lysozyme.htm
- ↑ Lysozyme. 2010. Citizendium.org. http://en.citizendium.org/wiki/Lysozyme
- ↑ Lysozyme. 2008. Lysozyme.co.uk. http://lysozyme.co.uk/
- ↑ Lysozyme, 2008. Lysozyme.co.uk. http://lysozyme.co.uk/
- ↑ Bugg, T. 1997. An Introduction to Enzyme and Coenzyme Chemistry. Blackwell Science Ltd., Oxford
- ↑ 1967. Proc R Soc Lond B Bio 167 (1009): 389–401.
- ↑ Lysozyme, 2008. Lysozyme.co.uk. http://lysozyme.co.uk/
- ↑ Pratt, C.W., Voet, D., Voet, J.G. Fundamentals of Biochemistry - Life at the Molecular Level - Third Edition. Voet, Voet and Pratt, 2008.
- ↑ Image from: http://www.google.com/imgres?imgurl=http://www.vuw.ac.nz/staff/paul_teesdale-spittle/essentials/chapter-6/pics-and-strucs/lysozyme-mech.gif&imgrefurl=http://www.vuw.ac.nz/staff/paul_teesdale-spittle/essentials/chapter-6/proteins/lysozyme.htm&usg=__ormapG4XKg-tR5GrMSOdSMTV4vE=&h=603&w=801&sz=7&hl=en&start=17&zoom=1&tbnid=nvr9gvFrUILDkM:&tbnh=143&tbnw=189&prev=/images%3Fq%3DThe%2Blysozyme%2Breaction%2Bmechanism%26um%3D1%26hl%3Den%26sa%3DN%26biw%3D1280%26bih%3D647%26tbs%3Disch:10%2C304&um=1&itbs=1&iact=hc&vpx=521&vpy=349&dur=448&hovh=191&hovw=254&tx=140&ty=48&ei=JQ_LTPKzLIjCsAPkzt2KDg&oei=IA_LTP74OsG78gapm-GFAQ&esq=2&page=2&ndsp=18&ved=1t:429,r:2,s:17&biw=1280&bih=647
- ↑ http://mcdb-webarchive.mcdb.ucsb.edu/sears/biochemistry/tw-enz/lysozyme/HEWL/lysozyme-overview.htm
- ↑ http://www.worthington-biochem.com/ly/default.html