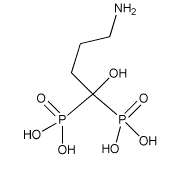
Alendronate
From Proteopedia
| Line 18: | Line 18: | ||
Alendronate is an aminobisphosphonate with a nonhydrolyzable P-C-P. Alendronate generally affects the activity of osteoclasts in bone. Osteoclasts are responsible for breaking down bone and also for bone resorption (losing bone substance). <ref>http://www.medterms.com/script/main/art.asp?articlekey=11794</ref> When alendronate is present, bone resorption is inhibited and bone breakdown is diminished. | Alendronate is an aminobisphosphonate with a nonhydrolyzable P-C-P. Alendronate generally affects the activity of osteoclasts in bone. Osteoclasts are responsible for breaking down bone and also for bone resorption (losing bone substance). <ref>http://www.medterms.com/script/main/art.asp?articlekey=11794</ref> When alendronate is present, bone resorption is inhibited and bone breakdown is diminished. | ||
| - | |||
== Target Proteins and Bone == | == Target Proteins and Bone == | ||
| Line 33: | Line 32: | ||
<Structure load='2f92' size='450' frame='true' align='right' caption='Shown: The asymmetric subunit of FPPS, not the assumed biological molecule.' scene='Insert optional scene name here' /> | <Structure load='2f92' size='450' frame='true' align='right' caption='Shown: The asymmetric subunit of FPPS, not the assumed biological molecule.' scene='Insert optional scene name here' /> | ||
| - | <scene name='Sandbox_59/Fpps/2'>FPPS</scene> is considered the main target protein of aminobisphosphonates (including alendronate). | + | <scene name='Sandbox_59/Fpps/2'>FPPS</scene> is considered the main target protein of aminobisphosphonates (including alendronate). FPPS is the prenyl transferase in the HMG-CoA Reductase Pathway, which forms cholesterol (sterols and terpenoids) from acetyl CoA. It reacts with 3-isopentenyl pyrophosphate. |
| - | + | The identified mechanism of alendronate on FPPS begins with the absorption of the alendronate into the osteoclast. Once in the osteoclast, the <scene name='Sandbox_59/Active_site_ligand_space_fill/2'>alendronate binds</scene> to the active site of FPPS.<ref>Fisher et. all. ''Alendronate mechanism of action: geranylgeraniol, an intermediate in the mevalonate pathway, prevents inhibition of osteoclast formation, bone resorption, and kinase activation in vitro''.Cell Biology, Vol. 96, pp. 133–138, January 1999</ref> | |
| - | <scene name='Sandbox_59/ | + | |
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| + | <scene name='Sandbox_59/Active_site_ligand_space_fill/2'>alendronate binds</scene> | ||
| + | <scene name='Sandbox_59/Active_site_ligand/2'>active site bound sticks</scene> | ||
<scene name='Sandbox_59/Fpps_bound_phosphate/2'>FPPS bound phosphate</scene> | <scene name='Sandbox_59/Fpps_bound_phosphate/2'>FPPS bound phosphate</scene> | ||
Revision as of 10:28, 11 March 2011
| Please do NOT make changes to this Sandbox. Sandboxes 30-60 are reserved for use by Biochemistry 410 & 412 at Messiah College taught by Dr. Hannah Tims during Fall 2012 and Spring 2013. |
Contents |
Alendronate (Fosamax®)
Alendronate is commonly known for its use in treatment and prevention of osteoporosis in postmenopausal women and men, but is also used to treat Paget's disease (disease that results in deformed and enlarged bones).[1] Alendronate belongs to the class of nitrogen-containing bisphosphonates, which are inorganic pyrophosphate analogues.
History of Bisphosphonates
Bisphosphonates were first synthesized in Germany in 1865, but were not studied biologically until 1968. In the interim time, they were used in the textile and fertilizer industries due to their apparent inhibitory effect on calcium carbonate. However, in 1968, a group in Switzerland found inorganic pyrophosphates in urine and plasma. In vitro testing of these molecules revealed that they inhibited calcium phosphate precipitation and dissolution, but were destroyed in vivo by phosphatases. These results led to the discovery of bisphosphonates, as they reacted in the prescribed manner.[2]
Sodium alendronate was first marketed in 1994 as Fosamax® by Merck pharmaceutical. In 2008, Merck lost their U.S. patent on alendronate, allowing Barr Pharmaceuticals and Teva Pharmaceuticals USA to begin marketing generic forms of sodium alendronate. Other brand names for the drug include Fosamax+D®, Adronat, Alendros, Arendal, and Onclast.[3]
Structure and General Function
Alendronate is an aminobisphosphonate with a nonhydrolyzable P-C-P. Alendronate generally affects the activity of osteoclasts in bone. Osteoclasts are responsible for breaking down bone and also for bone resorption (losing bone substance). [5] When alendronate is present, bone resorption is inhibited and bone breakdown is diminished.
Target Proteins and Bone
Alendronate not only targets several proteins, but also directly binds to a bone mineral (hydroxyapatite), which causes the inhibition of bone resorption. The mechanism for this has not been elucidated.[6][7]
Protein-Tyrosine-Phosphatases (PTP)
Protein-Tyrosine-Phosphatses (PTPs) are reported to have an effect on osteoclast formation and function.[8] Initial reports indicated that alendronate inhibited several types of PTPs, though specific mechanisms are not known. The data does suggest that alendronate works as an antagonist, which means that, when bound, the alendronate does not elicit a response from the protein but rather disallows the binding of an agonist which would cause a change (likely activating) in the protein.[9][10][11] A is given, so the binding site can be seen.
Farnesyl Pyrophosphate Synthase (FPPS)
|
is considered the main target protein of aminobisphosphonates (including alendronate). FPPS is the prenyl transferase in the HMG-CoA Reductase Pathway, which forms cholesterol (sterols and terpenoids) from acetyl CoA. It reacts with 3-isopentenyl pyrophosphate. The identified mechanism of alendronate on FPPS begins with the absorption of the alendronate into the osteoclast. Once in the osteoclast, the to the active site of FPPS.[12]
FPPS Natural Substrates
Isopentenyl pyrophosphate (IPP) actually binds to and stabilizes the alendronate-FPPS complex, rather than competing with the inhibitor.
Side affects of Drug
References
- ↑ http://www.ncbi.nlm.nih.gov/pubmedhealth/PMH0000018/
- ↑ http://www.ncbi.nlm.nih.gov/pmc/articles/PMC138713/?tool=pmcentrez
- ↑ http://www.drugbank.ca/drugs/DB00630
- ↑ Image from: http://pharmacy-and-drugs.com
- ↑ http://www.medterms.com/script/main/art.asp?articlekey=11794
- ↑ http://www.ncbi.nlm.nih.gov/pubmed/20209564
- ↑ http://www.ncbi.nlm.nih.gov/pubmed/16046206
- ↑
- ↑ http://www.drugbank.ca/drugs/DB00630
- ↑ http://www.ncbi.nlm.nih.gov/pubmed/8610169
- ↑ http://www.ncbi.nlm.nih.gov/pubmed/9310349
- ↑ Fisher et. all. Alendronate mechanism of action: geranylgeraniol, an intermediate in the mevalonate pathway, prevents inhibition of osteoclast formation, bone resorption, and kinase activation in vitro.Cell Biology, Vol. 96, pp. 133–138, January 1999
- ↑ Image from: http://reference.findtarget.com