Sandbox Reserved 348
From Proteopedia
m (Tweak 3D structures section) |
(Scene edits, added regulation section.) |
||
| Line 3: | Line 3: | ||
<!-- PLEASE ADD YOUR CONTENT BELOW HERE --> | <!-- PLEASE ADD YOUR CONTENT BELOW HERE --> | ||
| - | {{ STRUCTURE_1ppb | PDB=1ppb | SCENE=Sandbox_Reserved_348/Ligand/ | + | {{ STRUCTURE_1ppb | PDB=1ppb | SCENE=Sandbox_Reserved_348/Ligand/4 }} |
[[Image:Thrombin_in_Coagulation.png|thumb|left|300px|The role of thrombin and prothrombin in [http://en.wikipedia.org/wiki/Coagulation coagulation].]] | [[Image:Thrombin_in_Coagulation.png|thumb|left|300px|The role of thrombin and prothrombin in [http://en.wikipedia.org/wiki/Coagulation coagulation].]] | ||
| - | Thrombin is a [[trypsin]]-like [[ | + | Thrombin is a [[trypsin]]-like [[serine protease]] which is best known for its role in blood clotting. In humans, the F2 gene codes for prothrombin, which is also known as Coagulation Factor II.<ref name="Human genes encoding prothrombin and ceruloplasmin map to 11p11-q12 and 3q21-24, respectively.">PMID:3474786</ref><ref name="Nucleotide sequence of the gene for human prothrombin.">PMID:2825773</ref> Clevage of prothrombin to form activated α-thrombin is a key step in the final common pathway of blood clotting, because clevage by thrombin activates several factors in blood clotting, especially [[fibrin]], [[factor XIII]], and [[protein C]].<ref name="Thrombin interactions.">PMID:12970119</ref> |
__TOC__ | __TOC__ | ||
==Structure== | ==Structure== | ||
| - | Thrombin is comprised of two chains, often referred to as the <scene name='Sandbox_Reserved_348/Small_subunit/ | + | Thrombin is comprised of two chains, often referred to as the <scene name='Sandbox_Reserved_348/Small_subunit/3'>short chain</scene> and the <scene name='Sandbox_Reserved_348/Large_subunit/3'>long chain</scene>. All known functional epitopes are found on the long chain. There is one active site, which in the case of [[1ppb]] is occupied with <scene name='Sandbox_Reserved_348/Ligand/4'>D-Phe-Pro-Arg chloromethylketone</scene>.<ref name="The refined 1.9A crystal structure of human alpha-thrombin: interaction with D-Phe-Pro-Arg chloromethylketone and significance of the Tyr-Pro-Pro-Trp insertion segment.">PMID:2583108</ref> Additionally, there are three structural disulfide bonds. |
| + | |||
| + | While thrombin is described as a [[trypsin]]-like [[serine protease]], it is more specific than trypsin due to two exosites which bind the substrate at a point separate from the active site. These exosites also allow for more specific inhibition, since [[protein C]] and [[factor Xa]] have similar active sites but play very different roles in the clotting process.<ref name="Thrombin interactions."/> | ||
| + | |||
| + | ==Regulation== | ||
| + | |||
| + | Prothrombin is proteolytically activated to α-thrombin by [[factor Xa]]. α-Thrombin is permanently inactivated by [[antithrombin]], with [[heparin]] as a cofactor, and allosterically regulated by sodium ion concentration. [[Thrombomodulin]] inhibits clevage of fibrinogen to fibrin, but also enhances α-thrombin activity with respect to [[protein C]]. Since activated protein C proteolytically inactivates earlier steps in the chain, this effectively reverses the role of thrombin from coagulant to anticoagulant.<ref name="Thrombin interactions."/> | ||
==3D Structures== | ==3D Structures== | ||
| Line 26: | Line 32: | ||
*[[Trypsin]] | *[[Trypsin]] | ||
*[[Serine Protease]] | *[[Serine Protease]] | ||
| + | *[[Factor Xa]] | ||
==External Resources== | ==External Resources== | ||
Revision as of 06:39, 4 April 2011
| This Sandbox is Reserved from January 10, 2010, through April 10, 2011 for use in BCMB 307-Proteins course taught by Andrea Gorrell at the University of Northern British Columbia, Prince George, BC, Canada. |
To get started:
More help: Help:Editing |
| |||||||||
| 1ppb, resolution 1.92Å () | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Ligands: | |||||||||
| Activity: | Thrombin, with EC number 3.4.21.5 | ||||||||
| |||||||||
| |||||||||
| Resources: | FirstGlance, OCA, RCSB, PDBsum | ||||||||
| Coordinates: | save as pdb, mmCIF, xml | ||||||||
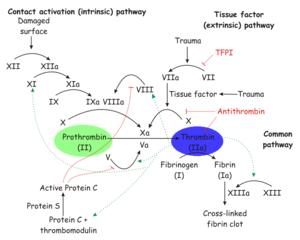
Thrombin is a trypsin-like serine protease which is best known for its role in blood clotting. In humans, the F2 gene codes for prothrombin, which is also known as Coagulation Factor II.[1][2] Clevage of prothrombin to form activated α-thrombin is a key step in the final common pathway of blood clotting, because clevage by thrombin activates several factors in blood clotting, especially fibrin, factor XIII, and protein C.[3]
Contents |
Structure
Thrombin is comprised of two chains, often referred to as the and the . All known functional epitopes are found on the long chain. There is one active site, which in the case of 1ppb is occupied with .[4] Additionally, there are three structural disulfide bonds.
While thrombin is described as a trypsin-like serine protease, it is more specific than trypsin due to two exosites which bind the substrate at a point separate from the active site. These exosites also allow for more specific inhibition, since protein C and factor Xa have similar active sites but play very different roles in the clotting process.[3]
Regulation
Prothrombin is proteolytically activated to α-thrombin by factor Xa. α-Thrombin is permanently inactivated by antithrombin, with heparin as a cofactor, and allosterically regulated by sodium ion concentration. Thrombomodulin inhibits clevage of fibrinogen to fibrin, but also enhances α-thrombin activity with respect to protein C. Since activated protein C proteolytically inactivates earlier steps in the chain, this effectively reverses the role of thrombin from coagulant to anticoagulant.[3]
3D Structures
α-Thrombin
Prothrombin
See Also
External Resources
- Thrombin at Wikipedia
- Serine protease at Wikipedia
- Fibrin Glue at Wikipedia
- Coagulation (blood clotting) at Wikipedia
- Hemophilia at Wikipedia
References
- ↑ Royle NJ, Irwin DM, Koschinsky ML, MacGillivray RT, Hamerton JL. Human genes encoding prothrombin and ceruloplasmin map to 11p11-q12 and 3q21-24, respectively. Somat Cell Mol Genet. 1987 May;13(3):285-92. PMID:3474786
- ↑ Degen SJ, Davie EW. Nucleotide sequence of the gene for human prothrombin. Biochemistry. 1987 Sep 22;26(19):6165-77. PMID:2825773
- ↑ 3.0 3.1 3.2 Di Cera E. Thrombin interactions. Chest. 2003 Sep;124(3 Suppl):11S-7S. PMID:12970119
- ↑ Bode W, Mayr I, Baumann U, Huber R, Stone SR, Hofsteenge J. The refined 1.9 A crystal structure of human alpha-thrombin: interaction with D-Phe-Pro-Arg chloromethylketone and significance of the Tyr-Pro-Pro-Trp insertion segment. EMBO J. 1989 Nov;8(11):3467-75. PMID:2583108

