Potassium Channel
From Proteopedia
| Line 1: | Line 1: | ||
<StructureSection load='' size='500' side='right' caption='Structure of the Potassium Channel, ([[2r9r]])' scene='Potassium_Channel/Opening/1'> | <StructureSection load='' size='500' side='right' caption='Structure of the Potassium Channel, ([[2r9r]])' scene='Potassium_Channel/Opening/1'> | ||
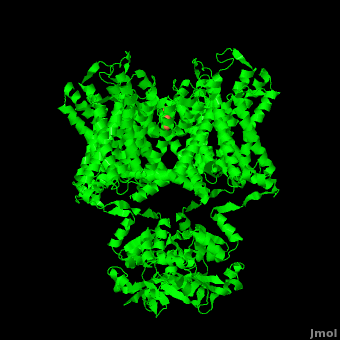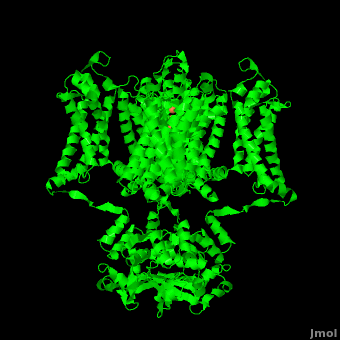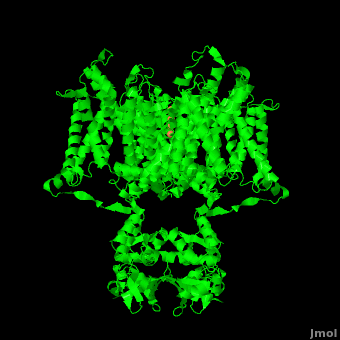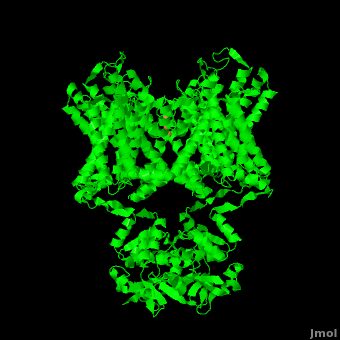
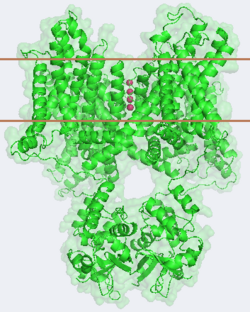
| - | [[Image:2r9r Picture Proteopedia2.png|250px|left]] [[Potassium Channel]]'''s''' control | + | [[Image:2r9r Picture Proteopedia2.png|250px|left]] [[Potassium Channel]]'''s''' control cell membrane electric potentials by selectively allowing diffusion of K<sup>+</sup> across the membrane.<ref name="Zhou">PMID: 11689936</ref> K<sup>+</sup> Channels extend across the cell membrane, a 40Å thick lipid bilayer which ions cannot cross.<ref name="Doyle">PMID: 9525859</ref> Potassium homeostasis is crucial for nearly all living cells, but is particularly important for the correct function of neurons. Neurons produce electrical impulses known as action potentials, allow for cellular communication processes like neurotransmitter release or to initiate intercellular processes muscle contraction. At the onset of an action potential, sodium ions flood across the plasma membrane of neurons via sodium channels. The change in polarity of the plasma membrane caused by the sodium ion influx inactivates sodium channels. Potassium channels subsequently open allowing the selective diffusion of K<sup>+</sup> ions across the plasma membrane, returning the membrane polarity to neutral. After the action potential has passed, channels recreate the high potassium concentration within the cell in preparation for the next stiumulus.<ref>PMID:12721618</ref> Mutations in voltage-gated potassium channel KCNC3 have been linked with [[Neurodevelopmental Disorders|neurodevelopmental disorders]] and neurodegeneration.<ref>PMID: 16501573</ref> |
| - | Potassium channels possess two traits that are seemingly mutually exclusive. Firstly, potassium channels have exquisite selectivity, with an amazing 10,000 fold selectivity for K<sup>+</sup> ions over sodium ions. Considering the only difference by which potassium ions can be differentiated from sodium ions is potassium ions’ 1.33Å Pauling radius vs. Sodium’s .95Å radius, the selectivity of potassium channels is remarkable.<ref name="Doyle"/> Second, despite its remarkable selectivity, potassium channels allow for the transfer of K<sup>+</sup> ions across the cell membrane at a rate of nearly 10<sup>8</sup> per second, nearly at the diffusion rate limit.<ref name="Long">PMID: 18004376</ref> Potassium channels are able to achieve these remarkable feats due to its amazing structural architecture contains several | + | Potassium channels possess two traits that are seemingly mutually exclusive. Firstly, potassium channels have exquisite selectivity, with an amazing 10,000 fold selectivity for K<sup>+</sup> ions over sodium ions. Considering the only difference by which potassium ions can be differentiated from sodium ions is potassium ions’ 1.33Å Pauling radius vs. Sodium’s .95Å radius, the selectivity of potassium channels is remarkable.<ref name="Doyle"/> Second, despite its remarkable selectivity, potassium channels allow for the transfer of K<sup>+</sup> ions across the cell membrane at a rate of nearly 10<sup>8</sup> per second, nearly at the diffusion rate limit.<ref name="Long">PMID: 18004376</ref> Potassium channels are able to achieve these remarkable feats due to its amazing structural architecture, which contains several features which not only can sense the voltage potential across a membrane, but also selectively ferry K<sup>+</sup> ions without any outside energy expenditure. |
The overall structure of the voltage gated potassium channel can be seen in the image at the left. It is comprised of 4 identical subunits and contains several key features which will be analyzed. Primarily, a <scene name='Potassium_Channel/Trans/3'>transmembrane region</scene> marked between the parallel lines in the figure. This region houses the <scene name='Potassium_Channel/Pore_opening/5'>channel pore</scene>, composed of interwoven helices in a teepee conformation, the all-important <scene name='Potassium_Channel/Selectivity_filter_opening/2'>“selectivity filter”</scene>, providing the channel with its remarkable 10,00 fold selectivity for K<sup>+</sup> ions over Na<sup>+</sup> ions and the <scene name='Potassium_Channel/Voltage_sensors_opening/4'>“voltage sensor”</scene> which is uses well placed arginine and acidic residues to determine the membrane polarity and open/close the channel in response.<ref name="Long"/> | The overall structure of the voltage gated potassium channel can be seen in the image at the left. It is comprised of 4 identical subunits and contains several key features which will be analyzed. Primarily, a <scene name='Potassium_Channel/Trans/3'>transmembrane region</scene> marked between the parallel lines in the figure. This region houses the <scene name='Potassium_Channel/Pore_opening/5'>channel pore</scene>, composed of interwoven helices in a teepee conformation, the all-important <scene name='Potassium_Channel/Selectivity_filter_opening/2'>“selectivity filter”</scene>, providing the channel with its remarkable 10,00 fold selectivity for K<sup>+</sup> ions over Na<sup>+</sup> ions and the <scene name='Potassium_Channel/Voltage_sensors_opening/4'>“voltage sensor”</scene> which is uses well placed arginine and acidic residues to determine the membrane polarity and open/close the channel in response.<ref name="Long"/> | ||
====Selectivity Filter and Pore==== | ====Selectivity Filter and Pore==== | ||
| - | It is instructive to follow the path of a potassium ion as it enters the cell through the <scene name='Potassium_Channel/Potassium_out/3'>potassium channel</scene>. Upon <scene name='Potassium_Channel/Into_pore/4'>entering the channel</scene>, the K<sup>+</sup> ion first comes into contact with the <scene name='Potassium_Channel/From_extra/4'>selectivity filter</scene>. The solved structure of the potassium channel by MacKinnon et al. revealed where the channels remarkable selectivity comes from. When entering the <scene name='Potassium_Channel/From_extra/3'>selectivity filter</scene>, K<sup>+</sup> ions are first dehydrated, shedding up to 8 waters. To stabilize these naked ions, <scene name='Potassium_Channel/Selectivity_side/1'>a number of carbonyl oxygens</scene> (<scene name='Potassium_Channel/Selectivity_side_labels/3'>Labels</scene>) bind the K<sup>+</sup> ions. The distance between K<sup>+</sup> ion and carbonyl oxygen is at <scene name='Potassium_Channel/Selectivity_side_size/1'>the perfect width</scene> to accommodate K<sup>+</sup> ions but not Na<sup>+</sup> ions which are too small. If a Na<sup>+</sup> ion were to lose | + | It is instructive to follow the path of a potassium ion as it enters the cell through the <scene name='Potassium_Channel/Potassium_out/3'>potassium channel</scene>. Upon <scene name='Potassium_Channel/Into_pore/4'>entering the channel</scene>, the K<sup>+</sup> ion first comes into contact with the <scene name='Potassium_Channel/From_extra/4'>selectivity filter</scene>. The solved structure of the potassium channel by MacKinnon et al. revealed where the channels remarkable selectivity comes from. When entering the <scene name='Potassium_Channel/From_extra/3'>selectivity filter</scene>, K<sup>+</sup> ions are first dehydrated, shedding up to 8 waters. To stabilize these naked ions, <scene name='Potassium_Channel/Selectivity_side/1'>a number of carbonyl oxygens</scene> (<scene name='Potassium_Channel/Selectivity_side_labels/3'>Labels</scene>) bind the K<sup>+</sup> ions. The distance between K<sup>+</sup> ion and carbonyl oxygen is at <scene name='Potassium_Channel/Selectivity_side_size/1'>the perfect width</scene> to accommodate K<sup>+</sup> ions but not Na<sup>+</sup>, ions which are too small. If a Na<sup>+</sup> ion were to lose its water shell, the carbonyl oxygens could not successfully stabilize it in its naked form and thus it is energetically unfavorable for a Na<sup>+</sup> ion to enter the channel. There is room within the selectivity filter for <scene name='Potassium_Channel/Selectivity_side_four/1'>four potassium ions</scene>. This, as it turns out, is crucial as the presence of the positive cations in close proximity to one another effectively pushes the potassium ions through the filter via electrostatic forces. This helps explain how the potassium channel can have such a rapid turnover rate.<ref name="Doyle"/> Also, the <scene name='Potassium_Channel/Selectivity_side_polarity/2'>natural polarity of the helices</scene>, with the <scene name='Potassium_Channel/Selectivity_side_polarity/3'>carbonyl oxygens pointing down the pore</scene>, helps pull the positively charged ions through the channel quickly. Compared to the <scene name='Potassium_Channel/High_filter/1'>high-concentration channel</scene> ([[1k4c]]), when exposed to a low concentration of potassium, the channel assumes a <scene name='Potassium_Channel/Low_con/3'>"low concentration" conformation</scene> ([[1k4d]]) which is sealed shut via interactions with water molecules.<ref name="Zhou"/> |
| - | The <scene name='Potassium_Channel/High_filter_broad/1'>selectivity filter</scene> only makes up | + | The <scene name='Potassium_Channel/High_filter_broad/1'>selectivity filter</scene> only makes up the beginning of the <scene name='Potassium_Channel/Full_pore/5'>channel pore</scene>. With the exception of the selectivity filter, the pore lining is <scene name='Potassium_Channel/Full_pore_hdryo/2'>mainly hydrophobic</scene>. This hydrophobic lining provides an inert surface over which the diffusing ion can slide unimpaired. Immediately following the selectivity filter is an <scene name='Potassium_Channel/Full_pore_h20/1'>aqueous cavity</scene> (<scene name='Potassium_Channel/Full_pore_h20_spin/1'>Spinning Model</scene>). K<sup>+</sup> ions, after passing through the filter, rehydrate in this cavity, helping overcome much of the energetic difficulty of having a positively charged cation within a hydrophobic membrane. At the bottom of the 34Å pore containing transmembrane region lies a number of <scene name='Potassium_Channel/High_filter_aromatic/2'>aromatic residues</scene> which help form a seal between the pore and the intracellular cytoplasm.<ref name="Doyle"/> |
====Voltage Sensor==== | ====Voltage Sensor==== | ||
| - | Channel pore opening is dependent on the membrane voltage, a characteristic that is “sensed” by the <scene name='Potassium_Channel/Voltage_sensors_opening/6'>voltage sensor</scene>. The voltage sensor is comprised of <scene name='Potassium_Channel/Voltage_helices/3'>six helices</scene>, S0, S1, S2, S3, S4, & S5. Negatively charged | + | Channel pore opening is dependent on the membrane voltage, a characteristic that is “sensed” by the <scene name='Potassium_Channel/Voltage_sensors_opening/6'>voltage sensor</scene>. The voltage sensor is comprised of <scene name='Potassium_Channel/Voltage_helices/3'>six helices</scene>, S0, S1, S2, S3, S4, & S5. Negatively charged sensor residues are either located in the <scene name='Potassium_Channel/Voltage_external/3'>external cluster</scene>, consisting of Glu 183 (in the [[2r9r]] structure) and Glu 226, or in the <scene name='Potassium_Channel/Voltage_internal/4'>internal cluster</scene> consisting of Glu 154, Glu 236, and Asp 259. The external cluster is exposed to solvent while the internal cluster is buried. <scene name='Potassium_Channel/Voltage_phe/2'>Phenylalanine 233</scene> acts as a separator between the two clusters.<ref name="Long"/> The 7 <scene name='Potassium_Channel/Voltage_phe/3'>positively charged residues</scene> of the voltage sensor are located on the S4 helix. Lys 302 and Arg 305 <scene name='Potassium_Channel/Voltage_lower_pos/1'>form hydrogen bonds</scene> with the internal negative cluster while Arginines 287, 290, 293, 296 and 299 are <scene name='Potassium_Channel/Voltage_solvent/1'>exposed to the extracellular solution</scene> (<scene name='Potassium_Channel/Voltage_sensors_arginines_over/2'>Overview</scene>). When the voltage sensor is exposed to a strong negative electric field in the intracellular membrane, the positive gating charges shift inward with the α-carbon of Arg 290 coming in close proximity to Phe 233. This shift effectively squeezes the pore shut, closing the intracellular-extracellular pathway. For a comparison see: The <scene name='Potassium_Channel/Open/1'>Open</scene> Channel vs. The <scene name='Potassium_Channel/Closed/1'>Closed</scene> ([[1k4c]]) Channel.<ref name="Long"/> Or view the morph of the <scene name='Potassium_Channel/Morph/3'>channel opening and closing</scene> (<scene name='Potassium_Channel/Morph/4'>Cartoon Design</scene>). |
Overall, Potassium channels are remarkable structures that allow for near diffusion limit transfer of molecules with sub-angstrom specificity. Our understanding of the structure of Potassium Channels has opened up the potential for [[Pharmaceutical drugs|therapeutic intervention]] into Potassium channel related diseases. | Overall, Potassium channels are remarkable structures that allow for near diffusion limit transfer of molecules with sub-angstrom specificity. Our understanding of the structure of Potassium Channels has opened up the potential for [[Pharmaceutical drugs|therapeutic intervention]] into Potassium channel related diseases. | ||
| Line 105: | Line 105: | ||
==References== | ==References== | ||
<references/> | <references/> | ||
| - | |||
__NOTOC__ | __NOTOC__ | ||
__NOEDITSECTION__ | __NOEDITSECTION__ | ||
Revision as of 18:27, 31 March 2011
| |||||||||||
Additional Structures of Potassium Channels
Potassium channels (KCh) are subdivided into voltage-gated KCh and calcium-dependent KCh. The latter are subdivided into high- (BK, LKCa), intermediate- and small-conductance KCh (human SK1, rat SK2, SKCa). The T1 domain is a highly conserved N-terminal domain which is responsible for driving the tetramerization of the KCh α subunit. The inward rectifier KCh (IRK) passes current more easily in the inward direction. MthK is a calcium-dependent KCh from Methanobacterium thermoautrophicum.
1wlj, 2wlh, 2wli, 2wlj, 2wlm, 2wln, 2wlo - MmKCh - Magnetospirillum magnetoacticum
2k44 – KCh voltage-sensor paddle domain – NMR
3e86 – BcKCh transmembrane domain – Bacillus cereus
3e8g, 3e89, 3e8b, 3e8h – BcKCh transmembrane domain +ions
2q67, 2q68, 2q69, 2q6a, 3ouf – BcKCh (mutant)
2ahy, 2ahz – BcKCh+ions
1bl8, 1f6g - SlKCh (mutant) - Streptomyces lividans
2qto - SlKCh
1j95 - SlKCh+K+tetrabutylammonium
1jvm - SlKCh (mutant)+Rb+tetrabutylammonium
1jq1, 1jq2 - SlKCh inner transmembrane segment - NMR
3ifx - SlKCh pore domain
2pnv – rKCh leucine zipper domain SKCa – rat
3lut - rKCh Kv1.2 (mutant)
1dsx - rKCh N-terminal (mutant)
1qdv, 1qdw - rKCh Kv1.2 N-terminal
1kn7 - rKCh Kv1.4 N-terminal (mutant) - NMR
1nn7 - rKCh Kv4.2 N-terminal T1 domain
3eau - rKCh beta2 +cortisone
3eb3, 3eb4 - rKCh beta2 (mutant) + cortisone
1a68, 1eod, 1eoe, 1eof, 3kvt - AcKCh Kv1.1 T1 domain (mutant) - Aplysia californica
1t1d - AcKCh Kv1.1 T1 domain
1b4g - hKCh inactivation domain - human - NMR
1byw - hKCh ERG N-terminal (mutant)
1ujl - hKCh ERG1 extracellular linker - NMR
2l1m, 2l4r - hKCh HERG - NMR
1s1g - hKCh Kv4.3 T1 domain
2ovc - hKCh Kv7.4 T1 domain
3bj4 - hKCh Kv7.1 C-termianl
3hfc, 3hfe - hKCh Kv7.1 (mutant)
1zxs - hKCh beta2
3co2, 1vp6 - MlotiK1 cyclic nucleotide binding domain - Mesorhizobium loti
1ho2, 1ho7 - KCh L45 segment - Drosophila melanogaster - NMR
2kyh - AeKCh voltage ensing domain - Aeropyrum pernix - NMR
2wll - KCh KIRBAC1.1 - Burkholderia pseudomalie
Potassium channel complex with protein
2nz0 – hKCh Kv4.3 N-terminal+KV channel interacting protein 1
2i2r - rKCh Kv4.3 N-terminal+KV channel interacting protein 1
1s6c - rKCh Kv4.2 N-terminal+KV channel interacting protein 1
2a79, 1qrq - rKCh Kv1.2 + beta2
2r9r - rKCh
1qx7 – rKCh SKCa+ calmodulin
1g4y – rKCh rSK2 calmodulin binding domain SKCa + calmodulin
1exb - rKCh Kv1.1 T1 domain + KV beta2 protein
2p7t - rKCh + FAV
3lnm - rKCh Kv2.1/KCh Kv1.2 (mutant)
3eff - mKCh + FAB - mouse
2w0f - mKCh + FAB + tetraoctylammonium
2hg5, 2h8p, 2hfe - KCh + mFAB
3f7v, 3f7y, 3fb5, 3fb7, 3fb8, 3gb7, 3hpl, 3iga, 3or6, 3or7, 1r3i, 1r3j, 1r3k,1r3l, 1s5h, 2atk, 2bob, 2boc, 2dwd, 2dwe, 2hvj, 2hvk, 2ih1, 2ih3, 2itc, 2itd, 2jk5, 2nlj - mKCh (mutant) + FAB
3f5w, 1zwi, [2hjf]] - mKCh (mutant) + antibody heavy+light chains
1k4c, 1k4d - SlKCh (mutant) + antibody heavy+light chains
2a0l - AeKCh + FV
1orq - AeKCh (mutant) + FAB
2a9h - SlKCh (mutant) + charybdotoxin
Inward rectifier KCh
1u4f,3agw - mIRK 2 cytoplasmic domain
2gix - mIRK 2 cytoplasmic domain (mutant)
2e4f - mIRK 2 fragment
1n9p, 1u4e - mIRK 1 cytoplasmic domain
3k6n - mIRK 1 cytoplasmic domain (mutant)
1xl4, 1xl6, 2wlk, 2x6a, 2x6b, 2x6c - MmIRK KIRBAC3.1
1p7b - BpIRK C-terminal
MthK
1kxd - MthK RCK domain + Cd - Methanobacterium thermoautrophicum
2ogu, 2fy8, 2aej, 2aem, 1lnq - MthK RCK domain
2aef - MthK RCK domain + Ca
BK channel
3mt5 - hBK cytoplasmic domain
1jo6 - BK beta 2 N-terminal KCNMB2 encoded LKCa - NMR
Additional Resources
For Additional Information, See: Membrane Channels & Pumps
References
- ↑ 1.0 1.1 Zhou Y, Morais-Cabral JH, Kaufman A, MacKinnon R. Chemistry of ion coordination and hydration revealed by a K+ channel-Fab complex at 2.0 A resolution. Nature. 2001 Nov 1;414(6859):43-8. PMID:11689936 doi:http://dx.doi.org/10.1038/35102009
- ↑ 2.0 2.1 2.2 2.3 Doyle DA, Morais Cabral J, Pfuetzner RA, Kuo A, Gulbis JM, Cohen SL, Chait BT, MacKinnon R. The structure of the potassium channel: molecular basis of K+ conduction and selectivity. Science. 1998 Apr 3;280(5360):69-77. PMID:9525859
- ↑ Jiang Y, Lee A, Chen J, Ruta V, Cadene M, Chait BT, MacKinnon R. X-ray structure of a voltage-dependent K+ channel. Nature. 2003 May 1;423(6935):33-41. PMID:12721618 doi:http://dx.doi.org/10.1038/nature01580
- ↑ Waters MF, Minassian NA, Stevanin G, Figueroa KP, Bannister JP, Nolte D, Mock AF, Evidente VG, Fee DB, Muller U, Durr A, Brice A, Papazian DM, Pulst SM. Mutations in voltage-gated potassium channel KCNC3 cause degenerative and developmental central nervous system phenotypes. Nat Genet. 2006 Apr;38(4):447-51. Epub 2006 Feb 26. PMID:16501573 doi:ng1758
- ↑ 5.0 5.1 5.2 5.3 Long SB, Tao X, Campbell EB, MacKinnon R. Atomic structure of a voltage-dependent K+ channel in a lipid membrane-like environment. Nature. 2007 Nov 15;450(7168):376-82. PMID:18004376 doi:http://dx.doi.org/10.1038/nature06265
Proteopedia Page Contributors and Editors (what is this?)
Michal Harel, David Canner, Joel L. Sussman, Alexander Berchansky, Ann Taylor