Sandbox Reserved 195
From Proteopedia
| Line 69: | Line 69: | ||
| - | Biological work on RNase S helped scientists determine the first 3-dimensional structure of a protein-nucleic acid complex. Additionally, RNase S provided one of the first demonstrations of a crystalline enzyme acting as an active catalyst. Investigations of RNase S have also led to many technological advancements, including the following: substrate leash amplification, fusion protein systems, and protein ubiquitination <ref name="Raines"/ref>. RNase A and its derivatives have also been linked to have anti-cancer attributes. | + | Biological work on RNase S helped scientists determine the first 3-dimensional structure of a protein-nucleic acid complex. Additionally, RNase S provided one of the first demonstrations of a crystalline enzyme acting as an active catalyst. Investigations of RNase S have also led to many technological advancements, including the following: substrate leash amplification, fusion protein systems, and protein ubiquitination <ref name="Raines" /ref>. RNase A and its derivatives have also been linked to have anti-cancer attributes. |
| + | |||
[[References ]] | [[References ]] | ||
| Line 76: | Line 77: | ||
Other resources: | Other resources: | ||
| + | |||
<ref name='Wikipedia'/> Wikipedia article on RNase A: http://en.wikipedia.org/wiki/Rnase_A | <ref name='Wikipedia'/> Wikipedia article on RNase A: http://en.wikipedia.org/wiki/Rnase_A | ||
Revision as of 18:43, 31 March 2011
| This Sandbox is Reserved from Feb 02, 2011, through Jul 31, 2011 for use by the Biochemistry II class at the Butler University at Indianapolis, IN USA taught by R. Jeremy Johnson. This reservation includes Sandbox Reserved 191 through Sandbox Reserved 200. |
To get started:
More help: Help:Editing |
Ribonuclease S
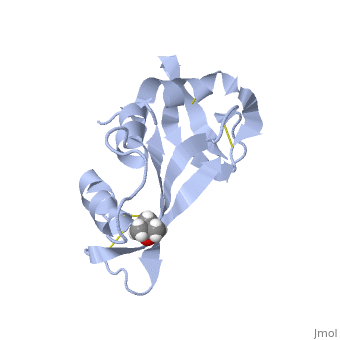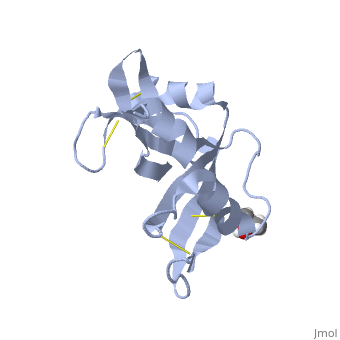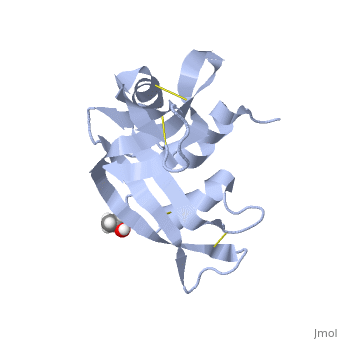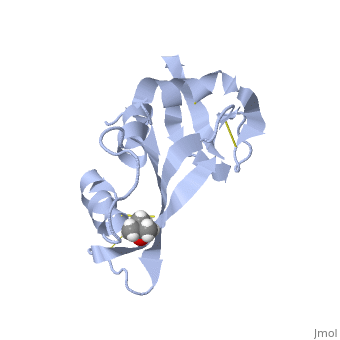
Ribonuclease S consists of two fragments of bovine Ribonuclease A, S peptide and S protein which make up the peptide-protein complex. RNase S has been studied to reveal mechanisms of protein folding by coupling folding and association. Studies of RNase S lead to greater interest in the investigation of RNase A and its role in biological systems. Through such studies, it has been suggested that RNase A and its oligomers could have anti-cancer effects, and a greater knowledge of the correlation between protein folding and enzyme activity.
|
RNase S is RNase A treated with subtilisin, which cleaves a single peptide bond. Consequently, RNase A and RNase S have very similar structures except for a few key areas, one being the cleavage site (residues 16-23)[1]. This contributes to a decrease in order in RNase S. The on RNase A is located between residues Ala 20 and Ser 21. Upon cleavage the fragments S peptide and S protein are identified giving RNase S its structure. consists of residues 1-20, and is largely responsible for proper folding. S protein is comprised of residues 21-124. Additionally, RNase A and RNase S have many conserved sequences. Glutamine 60 is not only conserved in and , but for the entire class of ribonucleases. Glu 60 is also interesting, because it is the only residue in an unfavorable position (Φ= -100, ψ= -130) as defined in the Ramachandran plot [2].
The of RNase S consists of residues His 12, Lys 41, Val 43, Asn 44, Thr 45, His 119, Phe 120, Asp 121, and Ser 123. Residues 1, 15-20, 21-23, and 124 could be removed without serious consequence to the structure or activity of RNase S. Residue 124 could also be removed from RNase A without compromising the structure. [3]
|
Hydrogen bonding and hydrophobic interactions play a major role in the structure of RNase S. [4] There are 84 water molecules present, with 8 of these specifically connecting S peptide and S protein. Some of these water molecules are also conserved throughout all RNase derivatives. Hydrophobic interactions between residues Phe 8, Met 13, His 12, Ala 4 are essential in holding the S Peptide in place. In addition to these residues, Asp 14 is also important in peptide-protein binding, as represented in the 2D picture. In the picture, it appears that the protein and peptide are not connected; this is because the bond cleavage between residues 20 and 21 of RNase A has already occurred. Additionally RNase S has a rigid hydrophobic core; one-third of the surface of the core is made up of the S peptide. The surrounding loops have more flexibility.
| Residue Asp 14 which aids in S peptide - S protein bond formation. |
Biological work on RNase S helped scientists determine the first 3-dimensional structure of a protein-nucleic acid complex. Additionally, RNase S provided one of the first demonstrations of a crystalline enzyme acting as an active catalyst. Investigations of RNase S have also led to many technological advancements, including the following: substrate leash amplification, fusion protein systems, and protein ubiquitination [1]