User:Luis E Ramirez-Tapia/T7 RNA polymerase
From Proteopedia
m (→Conformational Changes on T7 RNA Polymerase) |
(→Understanding the morph) |
||
| Line 37: | Line 37: | ||
</jmolButton> | </jmolButton> | ||
</jmol> | </jmol> | ||
| - | The first striking observation is the the<b> HUGE conformational change</b> of the <font color='magenta'>N-terminus part of the enzyme</font> | + | The first striking observation is the the<b> HUGE conformational change</b> of the <font color='magenta'>N-terminus part of the enzyme</font> and the <font color='orange'>helices C1-C2</font>. |
| - | The DNA with translucent color is our reference point and it is part of the intermediate state structure. The <font color='magenta'>N-terminus</font> rotates around 47º, the RNA transcript has 7 bases, still the enzyme has not reached its elongation conformation. The missing steps could be resolved if we morph the structures using the intermediate state structure and the elongation structure. I produced this <scene name='User:Luis_E_Ramirez-Tapia/Sandbox_3/T7wrongtransition/1'>CONFORMATIONAL CHANGE</scene>, however an interesting event happen. Could you see | + | The DNA with translucent color is our reference point and it is part of the intermediate state structure. The <font color='magenta'>N-terminus</font> rotates around 47º, the RNA transcript has 7 bases, still the enzyme has not reached its elongation conformation. The missing steps could be resolved if we morph the structures using the intermediate state structure and the elongation structure. I produced this <scene name='User:Luis_E_Ramirez-Tapia/Sandbox_3/T7wrongtransition/1'>CONFORMATIONAL CHANGE</scene>, however an interesting event happen. The <font color ="green">Subdomain H</font> (alfa-helices in green) is complete refolded. However there is a problem. Could you see it?<font color='red'> follow the green helices and you will see it</font>. Yes it is not a the real transition yet. Although there has been good advances in solving the correct transition [http://www.ncbi.nlm.nih.gov/pubmed/17472344 (2)], the optimal way, is by producing structures of the transitional complexes from 9 and 10 mer transcripts. Another approach will require the label of the enzyme with fluorophores, then using FRET we could calculate the distances and make a model of the correct transition. That is work in progress... |
Finally the morphs were produced using the energy minimization morphing software from the [http://molmovdb.mbb.yale.edu/molmovdb/morph/ Yale Morph Server], the structures that were used are the INITIATION STATE (PDB ID: 1qln), the INTERMIDATE STATE (PDB ID: 3e2e) (1) and the ELONGATION STATE (PDB ID:1msw). | Finally the morphs were produced using the energy minimization morphing software from the [http://molmovdb.mbb.yale.edu/molmovdb/morph/ Yale Morph Server], the structures that were used are the INITIATION STATE (PDB ID: 1qln), the INTERMIDATE STATE (PDB ID: 3e2e) (1) and the ELONGATION STATE (PDB ID:1msw). | ||
| + | , | ||
=References= | =References= | ||
Revision as of 00:31, 28 April 2011
One of the CBI Molecules being studied in the University of Massachusetts Amherst Chemistry-Biology Interface Program at UMass Amherst and on display at the Molecular Playground
| ||||||
|
Color code N-Terminus domain, Subdomain H, Helices C1 and C2, specificity loop, Non-template strand, template strand and the nascent RNA strand |
Contents |
Conformational Changes on T7 RNA Polymerase
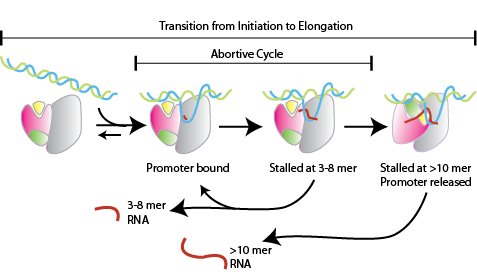
Transcription is a fundamental part of genetic regulation. The RNA polymerases that accomplish this function vary in structure, size and complexity, but must all carry out the same basic functions. The correct transcription of DNA to RNA depends of several factors and the complexity increases with the complexity of the organism. This makes the study of the transcriptional process complicate. The RNA polymerase of the bactereophage T7, is the perfect model for studying the transcription process. The main reason, it is a single unit enzyme that processes RNA with the same effectivity as the polymerase from higher organisms. Still much of the mechanism is unknown, for example, the mechanism of "abortive cycling" during the phase, (Figure 1.).
In this event the small RNA transcripts (less than 12 bases) fall from the complex. The abortive cycle will continue until the enzyme/DNA/RNA complex reach the phase, where a more stable enzyme/DNA/RNA complex is form. A mayor contributor of the stability of the complex is the formation of the . Another interesting observation, that could help to resolve the mechanism of abortive cycling, is a single point mutation at the , its substitution by a leucine decreases the amount of abortive products. Part of our research is focus on resolving the mechanism behind this mutation.
Understanding the morph
You can see the transition between the conformation and the complex by pressing the follow button. The first striking observation is the the HUGE conformational change of the N-terminus part of the enzyme and the helices C1-C2. The DNA with translucent color is our reference point and it is part of the intermediate state structure. The N-terminus rotates around 47º, the RNA transcript has 7 bases, still the enzyme has not reached its elongation conformation. The missing steps could be resolved if we morph the structures using the intermediate state structure and the elongation structure. I produced this , however an interesting event happen. The Subdomain H (alfa-helices in green) is complete refolded. However there is a problem. Could you see it? follow the green helices and you will see it. Yes it is not a the real transition yet. Although there has been good advances in solving the correct transition (2), the optimal way, is by producing structures of the transitional complexes from 9 and 10 mer transcripts. Another approach will require the label of the enzyme with fluorophores, then using FRET we could calculate the distances and make a model of the correct transition. That is work in progress... Finally the morphs were produced using the energy minimization morphing software from the Yale Morph Server, the structures that were used are the INITIATION STATE (PDB ID: 1qln), the INTERMIDATE STATE (PDB ID: 3e2e) (1) and the ELONGATION STATE (PDB ID:1msw). ,
References