This old version of Proteopedia is provided for student assignments while the new version is undergoing repairs. Content and edits done in this old version of Proteopedia after March 1, 2026 will eventually be lost when it is retired in about June of 2026.
Apply for new accounts at the new Proteopedia. Your logins will work in both the old and new versions.
Journal:JBIC:11
From Proteopedia
(Difference between revisions)

| Line 10: | Line 10: | ||
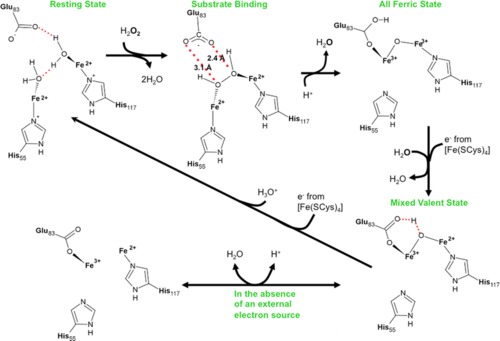
<scene name='Journal:JBIC:11/Cv/4'>Rubrerythrin peroxide binding site containing two iron atoms</scene> is formed by 2 adjacent monomers <font color='darkmagenta'><b>A (colored darkmagenta)</b></font> and <font color='magenta'><b>B (colored magenta)</b></font>. Rubrerythrin in the <scene name='Journal:JBIC:11/Cv/7'>reduced, resting (all-ferrous) state has two water molecules near the diiron center</scene> ([[3pwf]]). Rubrerythrin in the <scene name='Journal:JBIC:11/Cv/8'>fully oxidized all-ferric state</scene> ([[3mps]]), after 20 s exposure to peroxide, has μ-oxo bridge. Monomers of oxidized rubrerythrin <font color='skyblue'><b>A (colored skyblue)</b></font> and <font color='cyan'><b>B (colored cyan)</b></font>. This exposure <scene name='Journal:JBIC:11/Cv/9'>causes significant conformational changes of residues within the peroxide binding site</scene>. Movement of E114 upon oxidation of the all-ferrous diiron center to the fully oxidized all-ferric | <scene name='Journal:JBIC:11/Cv/4'>Rubrerythrin peroxide binding site containing two iron atoms</scene> is formed by 2 adjacent monomers <font color='darkmagenta'><b>A (colored darkmagenta)</b></font> and <font color='magenta'><b>B (colored magenta)</b></font>. Rubrerythrin in the <scene name='Journal:JBIC:11/Cv/7'>reduced, resting (all-ferrous) state has two water molecules near the diiron center</scene> ([[3pwf]]). Rubrerythrin in the <scene name='Journal:JBIC:11/Cv/8'>fully oxidized all-ferric state</scene> ([[3mps]]), after 20 s exposure to peroxide, has μ-oxo bridge. Monomers of oxidized rubrerythrin <font color='skyblue'><b>A (colored skyblue)</b></font> and <font color='cyan'><b>B (colored cyan)</b></font>. This exposure <scene name='Journal:JBIC:11/Cv/9'>causes significant conformational changes of residues within the peroxide binding site</scene>. Movement of E114 upon oxidation of the all-ferrous diiron center to the fully oxidized all-ferric | ||
state results in a <scene name='Journal:JBIC:11/Cv/10'>significant distortion of an alpha-helix</scene>. The <scene name='Journal:JBIC:11/Cv/11'>movement of backbone carbon atoms is over 1.7 A</scene> in some cases. Rubrerythrin was also exposed to peroxide for <scene name='Journal:JBIC:11/Exp/1'>10</scene> and 15 s and previously unobserved intermediates in the reaction cycle were identified. | state results in a <scene name='Journal:JBIC:11/Cv/10'>significant distortion of an alpha-helix</scene>. The <scene name='Journal:JBIC:11/Cv/11'>movement of backbone carbon atoms is over 1.7 A</scene> in some cases. Rubrerythrin was also exposed to peroxide for <scene name='Journal:JBIC:11/Exp/1'>10</scene> and 15 s and previously unobserved intermediates in the reaction cycle were identified. | ||
| - | (<scene name='Journal:JBIC:11/Exp/2'></scene>) | + | (<scene name='Journal:JBIC:11/Exp/2'>text</scene>) |
</StructureSection> | </StructureSection> | ||
<references/> | <references/> | ||
__NOEDITSECTION__ | __NOEDITSECTION__ | ||
Revision as of 09:12, 5 July 2011
| |||||||||||
- ↑ Dillard BD, Demick JM, Adams MW, Lanzilotta WN. A cryo-crystallographic time course for peroxide reduction by rubrerythrin from Pyrococcus furiosus. J Biol Inorg Chem. 2011 Jun 7. PMID:21647777 doi:10.1007/s00775-011-0795-6
This page complements a publication in scientific journals and is one of the Proteopedia's Interactive 3D Complement pages. For aditional details please see I3DC.