Journal:JBIC:11
From Proteopedia
(Difference between revisions)

| Line 10: | Line 10: | ||
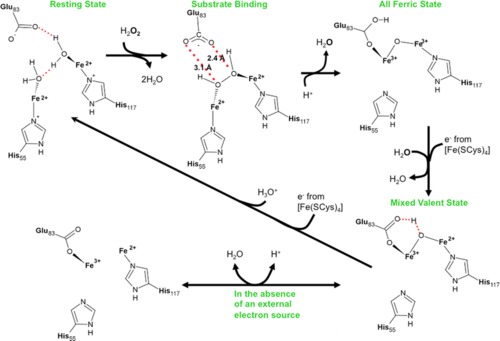
<scene name='Journal:JBIC:11/Cv/4'>Rubrerythrin peroxide binding site containing two iron atoms</scene> is formed by 2 adjacent monomers <font color='darkmagenta'><b>A (colored darkmagenta)</b></font> and <font color='magenta'><b>B (colored magenta)</b></font>. Rubrerythrin in the <scene name='Journal:JBIC:11/Cv/7'>reduced, resting (all-ferrous) state has two water molecules near the diiron center</scene> ([[3pwf]]). Rubrerythrin in the <scene name='Journal:JBIC:11/Cv/8'>fully oxidized all-ferric state</scene> ([[3mps]]), after 20 s exposure to peroxide, has μ-oxo bridge. Monomers of oxidized rubrerythrin <font color='skyblue'><b>A (colored skyblue)</b></font> and <font color='cyan'><b>B (colored cyan)</b></font>. This exposure <scene name='Journal:JBIC:11/Cv/9'>causes significant conformational changes of residues within the peroxide binding site</scene>. Movement of E114 upon oxidation of the all-ferrous diiron center to the fully oxidized all-ferric | <scene name='Journal:JBIC:11/Cv/4'>Rubrerythrin peroxide binding site containing two iron atoms</scene> is formed by 2 adjacent monomers <font color='darkmagenta'><b>A (colored darkmagenta)</b></font> and <font color='magenta'><b>B (colored magenta)</b></font>. Rubrerythrin in the <scene name='Journal:JBIC:11/Cv/7'>reduced, resting (all-ferrous) state has two water molecules near the diiron center</scene> ([[3pwf]]). Rubrerythrin in the <scene name='Journal:JBIC:11/Cv/8'>fully oxidized all-ferric state</scene> ([[3mps]]), after 20 s exposure to peroxide, has μ-oxo bridge. Monomers of oxidized rubrerythrin <font color='skyblue'><b>A (colored skyblue)</b></font> and <font color='cyan'><b>B (colored cyan)</b></font>. This exposure <scene name='Journal:JBIC:11/Cv/9'>causes significant conformational changes of residues within the peroxide binding site</scene>. Movement of E114 upon oxidation of the all-ferrous diiron center to the fully oxidized all-ferric | ||
state results in a <scene name='Journal:JBIC:11/Cv/10'>significant distortion of an alpha-helix</scene>. The <scene name='Journal:JBIC:11/Cv/11'>movement of backbone carbon atoms is over 1.7 A</scene> in some cases. Rubrerythrin was also exposed to peroxide for <scene name='Journal:JBIC:11/Exp/1'>10</scene> and 15 s and previously unobserved intermediates in the reaction cycle were identified. | state results in a <scene name='Journal:JBIC:11/Cv/10'>significant distortion of an alpha-helix</scene>. The <scene name='Journal:JBIC:11/Cv/11'>movement of backbone carbon atoms is over 1.7 A</scene> in some cases. Rubrerythrin was also exposed to peroxide for <scene name='Journal:JBIC:11/Exp/1'>10</scene> and 15 s and previously unobserved intermediates in the reaction cycle were identified. | ||
| - | (<scene name='Journal:JBIC:11/Exp/2'></scene>) | + | (<scene name='Journal:JBIC:11/Exp/2'>text</scene>) |
</StructureSection> | </StructureSection> | ||
<references/> | <references/> | ||
__NOEDITSECTION__ | __NOEDITSECTION__ | ||
Revision as of 09:12, 5 July 2011
| |||||||||||
- ↑ Dillard BD, Demick JM, Adams MW, Lanzilotta WN. A cryo-crystallographic time course for peroxide reduction by rubrerythrin from Pyrococcus furiosus. J Biol Inorg Chem. 2011 Jun 7. PMID:21647777 doi:10.1007/s00775-011-0795-6
This page complements a publication in scientific journals and is one of the Proteopedia's Interactive 3D Complement pages. For aditional details please see I3DC.