This old version of Proteopedia is provided for student assignments while the new version is undergoing repairs. Content and edits done in this old version of Proteopedia after March 1, 2026 will eventually be lost when it is retired in about June of 2026.
Apply for new accounts at the new Proteopedia. Your logins will work in both the old and new versions.
2iov
From Proteopedia
(New page: 200px<br /><applet load="2iov" size="350" color="white" frame="true" align="right" spinBox="true" caption="2iov, resolution 1.80Å" /> '''Bright-state structu...) |
|||
| Line 4: | Line 4: | ||
==Overview== | ==Overview== | ||
| - | RSFPs (reversibly switchable fluorescent proteins) may be repeatedly | + | RSFPs (reversibly switchable fluorescent proteins) may be repeatedly converted between a fluorescent and a non-fluorescent state by irradiation and have attracted widespread interest for many new applications. The RSFP Dronpa may be switched with blue light from a fluorescent state into a non-fluorescent state, and back again with UV light. To obtain insight into the underlying molecular mechanism of this switching, we have determined the crystal structure of the fluorescent equilibrium state of Dronpa. Its bicyclic chromophore is formed spontaneously from the Cys62-Tyr63-Gly64 tripeptide. In the fluorescent state, it adopts a slightly non-coplanar cis conformation within the interior of a typical GFP (green fluorescent protein) b-can fold. Dronpa shares some structural features with asFP595, another RSFP whose chromophore has previously been demonstrated to undergo a cis-trans isomerization upon photoswitching. Based on the structural comparison with asFP595, we have generated new Dronpa variants with an up to more than 1000-fold accelerated switching behaviour. The mutations which were introduced at position Val157 or Met159 apparently reduce the steric hindrance for a cis-trans isomerization of the chromophore, thus lowering the energy barrier for the blue light-driven on-to-off transition. The findings reported in the present study support the view that a cis-trans isomerization is one of the key events common to the switching mechanism in RSFPs. |
==About this Structure== | ==About this Structure== | ||
| Line 15: | Line 15: | ||
[[Category: Andresen, M.]] | [[Category: Andresen, M.]] | ||
[[Category: Eggeling, C.]] | [[Category: Eggeling, C.]] | ||
| - | [[Category: Hell, S | + | [[Category: Hell, S W.]] |
[[Category: Jakobs, S.]] | [[Category: Jakobs, S.]] | ||
| - | [[Category: Stiel, A | + | [[Category: Stiel, A C.]] |
[[Category: Trowitzsch, S.]] | [[Category: Trowitzsch, S.]] | ||
| - | [[Category: Wahl, M | + | [[Category: Wahl, M C.]] |
[[Category: Weber, G.]] | [[Category: Weber, G.]] | ||
[[Category: green-fluorescent protein-like protein]] | [[Category: green-fluorescent protein-like protein]] | ||
[[Category: reversibly switchable fluorescent protein]] | [[Category: reversibly switchable fluorescent protein]] | ||
| - | ''Page seeded by [http://oca.weizmann.ac.il/oca OCA ] on | + | ''Page seeded by [http://oca.weizmann.ac.il/oca OCA ] on Thu Feb 21 17:54:43 2008'' |
Revision as of 15:54, 21 February 2008
|
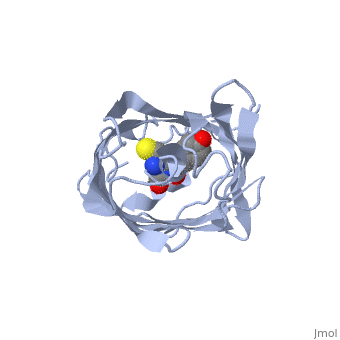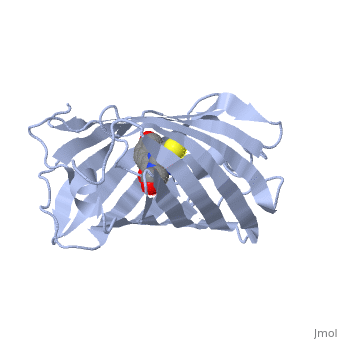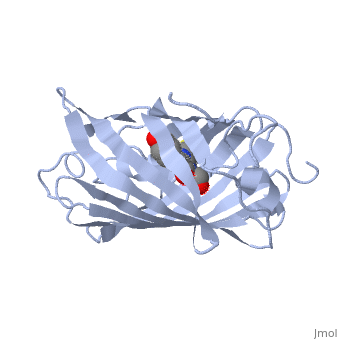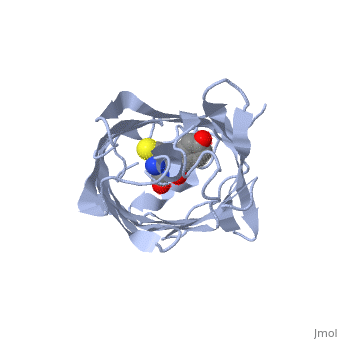
Bright-state structure of the reversibly switchable fluorescent protein Dronpa
Overview
RSFPs (reversibly switchable fluorescent proteins) may be repeatedly converted between a fluorescent and a non-fluorescent state by irradiation and have attracted widespread interest for many new applications. The RSFP Dronpa may be switched with blue light from a fluorescent state into a non-fluorescent state, and back again with UV light. To obtain insight into the underlying molecular mechanism of this switching, we have determined the crystal structure of the fluorescent equilibrium state of Dronpa. Its bicyclic chromophore is formed spontaneously from the Cys62-Tyr63-Gly64 tripeptide. In the fluorescent state, it adopts a slightly non-coplanar cis conformation within the interior of a typical GFP (green fluorescent protein) b-can fold. Dronpa shares some structural features with asFP595, another RSFP whose chromophore has previously been demonstrated to undergo a cis-trans isomerization upon photoswitching. Based on the structural comparison with asFP595, we have generated new Dronpa variants with an up to more than 1000-fold accelerated switching behaviour. The mutations which were introduced at position Val157 or Met159 apparently reduce the steric hindrance for a cis-trans isomerization of the chromophore, thus lowering the energy barrier for the blue light-driven on-to-off transition. The findings reported in the present study support the view that a cis-trans isomerization is one of the key events common to the switching mechanism in RSFPs.
About this Structure
2IOV is a Single protein structure of sequence from Echinophyllia sp. sc22. Full crystallographic information is available from OCA.
Reference
1.8 A bright-state structure of the reversibly switchable fluorescent protein Dronpa guides the generation of fast switching variants., Stiel AC, Trowitzsch S, Weber G, Andresen M, Eggeling C, Hell SW, Jakobs S, Wahl MC, Biochem J. 2007 Feb 15;402(1):35-42. PMID:17117927
Page seeded by OCA on Thu Feb 21 17:54:43 2008