Sandbox 39
From Proteopedia
| Line 2: | Line 2: | ||
{{Template: Oberholser Sandbox Reservation}} | {{Template: Oberholser Sandbox Reservation}} | ||
| - | < | + | <Structure load='9pap' size='300' frame='true' align='right' caption='Ribbon structure of Papain' scene='' /> |
=='''Papain'''== | =='''Papain'''== | ||
| Line 11: | Line 11: | ||
== Function == | == Function == | ||
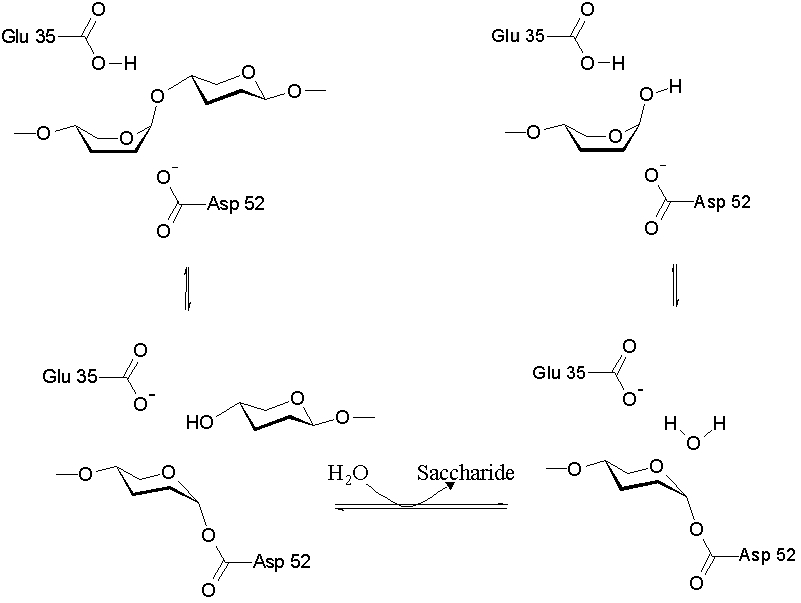
As a cysteine protease, Papain utilizes a nucleophilic cysteine thiol as part of its catalytic triad. Papain's Cys-25 is deprotonated by its His-159. The now nucleophilic Cys-25 attacks the carbonyl carbon of the peptide backbone, forming an acyl enzyme intermediate in which the peptide's amino terminal is free. Also in this step, His-159 is returned to its deprotonated form. The intermediate is then deacylated by a water molecule, and it releases the carboxyl terminal of the peptide to produce the product and regenerate the active enzyme. This entire mechanism is shown below: | As a cysteine protease, Papain utilizes a nucleophilic cysteine thiol as part of its catalytic triad. Papain's Cys-25 is deprotonated by its His-159. The now nucleophilic Cys-25 attacks the carbonyl carbon of the peptide backbone, forming an acyl enzyme intermediate in which the peptide's amino terminal is free. Also in this step, His-159 is returned to its deprotonated form. The intermediate is then deacylated by a water molecule, and it releases the carboxyl terminal of the peptide to produce the product and regenerate the active enzyme. This entire mechanism is shown below: | ||
| + | |||
[[Image:jrip.jpg]] | [[Image:jrip.jpg]] | ||
<ref>Image from: | <ref>Image from: | ||
| - | http://upload.wikimedia.org/wikipedia/commons/5/5c/Cysteinprotease_Reaktionsmechanismus.svg</ref> | + | http://upload.wikimedia.org/wikipedia/commons/5/5c/Cysteinprotease_Reaktionsmechanismus.svg</ref>]] |
<scene name='Sandbox_39/Hydrphobic_residues/1'>hydrophobic residues shown in yellow</scene> | <scene name='Sandbox_39/Hydrphobic_residues/1'>hydrophobic residues shown in yellow</scene> | ||
Revision as of 23:16, 8 November 2011
| Please do NOT make changes to this Sandbox. Sandboxes 30-60 are reserved for use by Biochemistry 410 & 412 at Messiah College taught by Dr. Hannah Tims during Fall 2012 and Spring 2013. |
|
Papain
Introduction
Papain (9PAP), also known as papaya proteinase I, is an enzyme found in unripe papaya fruit. A cysteine protease, it has been used to break down tough muscle fibers, and hence is often found in powdered meat tenderizers. It is collected from the fruit by scoring its skin and allowing the "sap" to seem out. The sap is then dried and purified.
Function
As a cysteine protease, Papain utilizes a nucleophilic cysteine thiol as part of its catalytic triad. Papain's Cys-25 is deprotonated by its His-159. The now nucleophilic Cys-25 attacks the carbonyl carbon of the peptide backbone, forming an acyl enzyme intermediate in which the peptide's amino terminal is free. Also in this step, His-159 is returned to its deprotonated form. The intermediate is then deacylated by a water molecule, and it releases the carboxyl terminal of the peptide to produce the product and regenerate the active enzyme. This entire mechanism is shown below:
 [1]]]
[1]]]
