This old version of Proteopedia is provided for student assignments while the new version is undergoing repairs. Content and edits done in this old version of Proteopedia after March 1, 2026 will eventually be lost when it is retired in about June of 2026.
Apply for new accounts at the new Proteopedia. Your logins will work in both the old and new versions.
Pertussis Toxin-ATP Complex
From Proteopedia
(Difference between revisions)
| Line 5: | Line 5: | ||
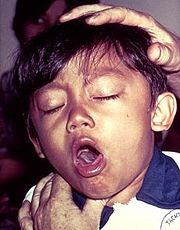
As of 2010, the worldwide incidence of whooping cough has been estimated to 48.5 million cases and nearly 295,000 deaths per year.<ref name=Bettiol>PMID: 20091541</ref> With that in mind, whooping cough can affect people of any age; however, before vaccines were available the disease was most common in infants and young children but now children are immunized and the high percentage of cases are seen among adolescents. | As of 2010, the worldwide incidence of whooping cough has been estimated to 48.5 million cases and nearly 295,000 deaths per year.<ref name=Bettiol>PMID: 20091541</ref> With that in mind, whooping cough can affect people of any age; however, before vaccines were available the disease was most common in infants and young children but now children are immunized and the high percentage of cases are seen among adolescents. | ||
| - | == | + | ==Structure== |
The [http://en.wikipedia.org/wiki/Pertussis_toxin pertussis toxin] has been characterized as being an AB toxin meaning that there are 2 subunits: A subunit possesses the enzyme activity and the B possesses it the receptor binding portion. PT in particular is an AB5 toxin consisting of a six-component protein complex, and the multiple subunits of the complex are not identical in composition. With that in mind, this protein is a hexamer containing a catalytic (S1) subunit that is tightly associated with the pentameric cell-binding component (B-oligomer). The S1 component is a single subunit <scene name='Pertussis_Toxin-ATP_Complex/Subunit_1/3'>S1 (chains A,G)</scene> while the B-oligomer is a pentamer composed of four types of subunits: <scene name='Pertussis_Toxin-ATP_Complex/Subunit_2/3'>S2 (chains B,H)</scene>, <scene name='Pertussis_Toxin-ATP_Complex/Subunit_3/5'>S3 (chains C,I)</scene>, two copies of <scene name='Pertussis_Toxin-ATP_Complex/Subunit_4/3'>S4 (chains D,E,J,K)</scene>, and <scene name='Pertussis_Toxin-ATP_Complex/Subunit_5/4'>S5 (chains F,L)</scene>.<ref name=Hazes>PMID: 8637000</ref> The overall structure of PT <scene name='Pertussis_Toxin-ATP_Complex/Whole_monomer/1'>multisubunit complex</scene>. These subunits are encoded by ptx genes, which are encoded on a large PT [http://en.wikipedia.org/wiki/Operon operon] that includes additional genes as well such as Pti genes. Together the PT and Pti proteins form the PT secretion complex and toxin itself.<ref name=Weiss>PMID: 8464913</ref> | The [http://en.wikipedia.org/wiki/Pertussis_toxin pertussis toxin] has been characterized as being an AB toxin meaning that there are 2 subunits: A subunit possesses the enzyme activity and the B possesses it the receptor binding portion. PT in particular is an AB5 toxin consisting of a six-component protein complex, and the multiple subunits of the complex are not identical in composition. With that in mind, this protein is a hexamer containing a catalytic (S1) subunit that is tightly associated with the pentameric cell-binding component (B-oligomer). The S1 component is a single subunit <scene name='Pertussis_Toxin-ATP_Complex/Subunit_1/3'>S1 (chains A,G)</scene> while the B-oligomer is a pentamer composed of four types of subunits: <scene name='Pertussis_Toxin-ATP_Complex/Subunit_2/3'>S2 (chains B,H)</scene>, <scene name='Pertussis_Toxin-ATP_Complex/Subunit_3/5'>S3 (chains C,I)</scene>, two copies of <scene name='Pertussis_Toxin-ATP_Complex/Subunit_4/3'>S4 (chains D,E,J,K)</scene>, and <scene name='Pertussis_Toxin-ATP_Complex/Subunit_5/4'>S5 (chains F,L)</scene>.<ref name=Hazes>PMID: 8637000</ref> The overall structure of PT <scene name='Pertussis_Toxin-ATP_Complex/Whole_monomer/1'>multisubunit complex</scene>. These subunits are encoded by ptx genes, which are encoded on a large PT [http://en.wikipedia.org/wiki/Operon operon] that includes additional genes as well such as Pti genes. Together the PT and Pti proteins form the PT secretion complex and toxin itself.<ref name=Weiss>PMID: 8464913</ref> | ||
==Pertussis Toxin activation== | ==Pertussis Toxin activation== | ||
| - | '''Pertussis Toxin''' by itself is harmless unless activated. From multiple studies, it has became clear that there is a direct interaction between [http://en.wikipedia.org/wiki/Adenosine_triphosphate Adenosine triphosphate] (ATP) and pertussis toxin which leads to activation. | + | '''Pertussis Toxin''' by itself is harmless unless activated. From multiple studies, it has became clear that there is a direct interaction between [http://en.wikipedia.org/wiki/Adenosine_triphosphate Adenosine triphosphate] (ATP) and pertussis toxin which leads to activation.<ref name=Hazes>PMID: 8637000</ref><ref name=Kaslow>PMID: 1612292</ref>The direct effect of ATP is to destabilize the interaction between the S1 subunit and the B-oligomer by binding to the B-oligomer.<ref name=Hazes>PMID: 8637000</ref> This interaction relaxes the toxin by facilitating the subsequent reduction of a disulphide bond in the S1 subunit. The main interaction that leads to the destabilization is the favorable hydrogen bonding and electrostatic interaction between the triphosphate moiety and five positively charged amino acids:<scene name='Pertussis_Toxin-ATP_Complex/5_amino_acid_interaction/4'>Arg S2-150, Arg S3-150, Arg S3-151, Arg S4b-69, and Lys S2-151</scene>. In contrast, the negatively charged carboxyl terminus of subunit S1 interacts unfavorably with the negative charges of the triphosphate moiety, causing a displacement of the C-terminal of <scene name='Pertussis_Toxin-ATP_Complex/Repulsion_of_subunit_s1/4'>Tyr 233:A and Phe 235:A</scene>therefore, the repulsion between the triphosphate moiety and the C terminus of subunit S1 forms the mechanism by which the interaction between S1 and the B-Oligomer is destabilized.<ref name=Hazes>PMID: 8637000</ref>The details of the [http://proteopedia.org/wiki/images/9/94/PT_ATP_complex.png protein-ATP interactions] can also be seen here.<ref name=Hazes>PMID: 8637000</ref> |
==Mechanism of pathogenesis== | ==Mechanism of pathogenesis== | ||
| Line 25: | Line 25: | ||
==Conclusion== | ==Conclusion== | ||
| - | This paper was significant since it gave a clear understanding of the PT activation as well as a better understanding to the pathogenesis of the toxin. The key features of this proposal is that ATP binding signals the arrival of the PT in the endoplasmic reticulum | + | This paper was significant since it gave a clear understanding of the PT activation as well as a better understanding to the pathogenesis of the toxin. The key features of this proposal is that ATP binding signals the arrival of the PT in the endoplasmic reticulum by acting as a molecular sensor.<ref name=Hazes>PMID: 8637000</ref> This detection of the PT in the ER at the same time triggers dissociation of the holotoxin prior to membrane translocation.<ref name=Hazes>PMID: 8637000</ref> Therefore, the dissociation is due to ATP binding destabilization and reduction by protein disulphide isomerase.<ref name=Hazes>PMID: 8637000</ref> |
| - | + | ||
</StructureSection> | </StructureSection> | ||
Revision as of 08:47, 12 November 2011
| |||||||||||
References
- ↑ 1.0 1.1 1.2 1.3 1.4 1.5 1.6 1.7 1.8 Hazes B, Boodhoo A, Cockle SA, Read RJ. Crystal structure of the pertussis toxin-ATP complex: a molecular sensor. J Mol Biol. 1996 May 17;258(4):661-71. PMID:8637000 doi:10.1006/jmbi.1996.0277
- ↑ Carbonetti NH. Immunomodulation in the pathogenesis of Bordetella pertussis infection and disease. Curr Opin Pharmacol. 2007 Jun;7(3):272-8. Epub 2007 Apr 5. PMID:17418639 doi:10.1016/j.coph.2006.12.004
- ↑ Bettiol S, Thompson MJ, Roberts NW, Perera R, Heneghan CJ, Harnden A. Symptomatic treatment of the cough in whooping cough. Cochrane Database Syst Rev. 2010 Jan 20;(1):CD003257. PMID:20091541 doi:10.1002/14651858.CD003257.pub3
- ↑ Weiss AA, Johnson FD, Burns DL. Molecular characterization of an operon required for pertussis toxin secretion. Proc Natl Acad Sci U S A. 1993 Apr 1;90(7):2970-4. PMID:8464913
- ↑ Kaslow HR, Burns DL. Pertussis toxin and target eukaryotic cells: binding, entry, and activation. FASEB J. 1992 Jun;6(9):2684-90. PMID:1612292