Sandbox 35
From Proteopedia
| Line 20: | Line 20: | ||
Papain. Lights. Camera. Action! | Papain. Lights. Camera. Action! | ||
| - | + | <StructureSection load='9pap' size='500' side='right' caption='Structure of HMG-CoA reductase (PDB entry [[9pap]])' scene=''> | |
| - | + | ||
| - | < | + | |
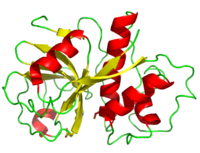
==Structure== | ==Structure== | ||
Papain's single polypeptide chain consists of 212 amino acid residues which fold to form a groove containing the active site between its two domains. Its | Papain's single polypeptide chain consists of 212 amino acid residues which fold to form a groove containing the active site between its two domains. Its | ||
| Line 28: | Line 26: | ||
| - | The <scene name='Sandbox_35/Active_site_papain/4'>active site</scene> primarily consist of three main residues | + | The <scene name='Sandbox_35/Active_site_papain/4'>active site</scene> primarily consist of three main residues Cys 25, His 159, and Asn 175 holding resemblance to the catalytic triad of chymotrypsin <ref>PMID: 8140097</ref><ref>PMID: 2397208</ref>. However, growing studies are showing that the mechanism behind catalysis may actually involve a double catalytic site - consisting of Cys 25- His 159- Asn 175 ''and'' Cys 25- His 159- |
| - | <scene name='Sandbox_35/Active_site_papain/5'>Asp 158</scene>! It is postulated that "a two-state mechanism" takes place instead of a "single steric mechanism." <ref>PMID: 8140097</ref> In addition, replacement of Asn 175 with other residues such as Ala mutants, reveals a decrease in kcat (less efficiency). Despite this, the rate of hydrolysis is still significantly larger than non-catalytic rates, suggesting a less essential role | + | <scene name='Sandbox_35/Active_site_papain/5'>Asp 158</scene>! It is postulated that "a two-state mechanism" takes place instead of a "single steric mechanism." <ref>PMID: 8140097</ref> In addition, replacement of Asn 175 with other residues such as Ala mutants, reveals a decrease in kcat (less efficiency). Despite this, the rate of hydrolysis is still significantly larger than non-catalytic rates, suggesting a less essential role Asn 175 plays than originally thought. Building on these observations, alteration to the 175 side chain results in less thermal stability lending thought that Asn 175 plays a more structural conservative rather than catalytic role. <ref>[http://www.jbc.org/content/270/28/16645.abstract] The Journal of Biological Chemistry </ref> |
| Line 43: | Line 41: | ||
<scene name='Sandbox_35/Cathepsin_l_specific_inhibitor/3'>Cathepsin L specific inhibitor</scene> is part of a series known as CLIK inhibitors and was used on papain as an assessment of inhibition specificity for cathepsin enzymes. Structural differences between Papain-CLIK 148 complex and original papain is not very drastic. Minute changes result primarily from alterations in surface proteins except where a covalent bond is formed between the C2 on <scene name='Sandbox_35/Clik_cys/1'>CLIK 148 and Cys 25 residue</scene>. The primary <scene name='Sandbox_35/Cathepsin_interaction/3'>interactions</scene> between pseudo-substrate/inhibitor and papain were non-water hydrogen bonds and mostly hydrophobic interactions. CLIK 148's binding to the active site of papain is in a non-substrate mode with the main site showing pyrimidine ring interaction between <scene name='Sandbox_35/Clik_trp_177/1'>Trp 177 and CLIK 148</scene>. Hydrogen bonding is observed between the oxygens in <scene name='Sandbox_35/Clik_gly_gln/1'>CLIK 148 to Gln 19 and Gly 66 residues</scene>. Moreover, a water molecule has been observed to be near the His 159 residue enabling greater hydrogen bonding, once again highlighting solvents role in stability. <ref>PMID: 10600517</ref> | <scene name='Sandbox_35/Cathepsin_l_specific_inhibitor/3'>Cathepsin L specific inhibitor</scene> is part of a series known as CLIK inhibitors and was used on papain as an assessment of inhibition specificity for cathepsin enzymes. Structural differences between Papain-CLIK 148 complex and original papain is not very drastic. Minute changes result primarily from alterations in surface proteins except where a covalent bond is formed between the C2 on <scene name='Sandbox_35/Clik_cys/1'>CLIK 148 and Cys 25 residue</scene>. The primary <scene name='Sandbox_35/Cathepsin_interaction/3'>interactions</scene> between pseudo-substrate/inhibitor and papain were non-water hydrogen bonds and mostly hydrophobic interactions. CLIK 148's binding to the active site of papain is in a non-substrate mode with the main site showing pyrimidine ring interaction between <scene name='Sandbox_35/Clik_trp_177/1'>Trp 177 and CLIK 148</scene>. Hydrogen bonding is observed between the oxygens in <scene name='Sandbox_35/Clik_gly_gln/1'>CLIK 148 to Gln 19 and Gly 66 residues</scene>. Moreover, a water molecule has been observed to be near the His 159 residue enabling greater hydrogen bonding, once again highlighting solvents role in stability. <ref>PMID: 10600517</ref> | ||
| - | + | </StructureSection> | |
==Catalytic Mechanism== | ==Catalytic Mechanism== | ||
Revision as of 13:24, 14 November 2011
| Please do NOT make changes to this Sandbox. Sandboxes 30-60 are reserved for use by Biochemistry 410 & 412 at Messiah College taught by Dr. Hannah Tims during Fall 2012 and Spring 2013. |
Contents |
Papain
Introduction
DID YOU KNOW?
. Meat tenderizer. Old time home remedy for insect, jellyfish, and stingray stings[1]. Who would have thought that a sulfhydryl protease from the latex of the papaya fruit, Carica papaya and Vasconcellea cundinamarcensis would have such a practical application beyond proteopedia?
This protease belongs to an extended family of aminopeptidases, dipeptidyl peptidases, endopeptidases, and other enzymes having both exo- and endo-peptidase activity. The inactivated zymogen with N-terminal propeptide regions - providing stability in alkaline environments and enabling proper folding - is activated through removal of the propeptide regions. [2][3]
Papain. Lights. Camera. Action!
| |||||||||||
Catalytic Mechanism
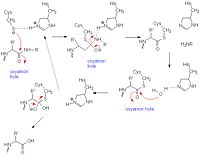
Papain's catalytic mechanism is like serine proteases. Its catalytic triad of residues Cys 25- His159- Arg-175 appear to work with a fourth residue, Gln-19, suspected to be involved in oxyanion hole formation. When a peptide binds to the active site, His-159 deprotonates Cys-25 which in turn attacks the substrate carbonyl carbon. The oxyanion hole then stabilizes the resulting covalent, tetrahedral intermediate. Subsequently, nitrogen in the peptide bond is protonated by His-159 (acting as an acid). This action frees the C-terminal portion of the peptide so that it is released. The entrance of water into the active site then attacks the carbonyl carbon while it is deprotonated by His-159, resulting in another tetrahedral covalent intermediate once again stabilized through the oxyanion hole. At the end, carbonyl reformation and the Cys-25 sulfur action as the leaving group releases the N-terminal portion of the peptide. The enzyme is regenerated for the cycle to begin again. [16]
Fun Trivia
Remember the 2002 SARS (Severe Acute Respiratory Syndrome) epidemic that placed global health, particularly in Southeast Asia, in a precarious state? On-going research is happening to further understand the mechanisms of this coronavirus, so that future steps can be taken for prevention. Its been found that the replication of RNA for this virus is mediated by two viral proteases that have many papain-like characteristics! [17]
References
I realize that there are repetitions in my citations. With PUBmed short cut syntax, I just couldn't figure out how to not repeat source in references; this being the least important, I've decided to leave this accordingly.
- ↑ [1] Ameridan International
- ↑ Rawlings ND, Barrett AJ. Families of cysteine peptidases. Methods Enzymol. 1994;244:461-86. PMID:7845226
- ↑ Yamamoto Y, Kurata M, Watabe S, Murakami R, Takahashi SY. Novel cysteine proteinase inhibitors homologous to the proregions of cysteine proteinases. Curr Protein Pept Sci. 2002 Apr;3(2):231-8. PMID:12188906
- ↑ [2]9PAP PDB
- ↑ Kamphuis IG, Kalk KH, Swarte MB, Drenth J. Structure of papain refined at 1.65 A resolution. J Mol Biol. 1984 Oct 25;179(2):233-56. PMID:6502713
- ↑ Wang J, Xiang YF, Lim C. The double catalytic triad, Cys25-His159-Asp158 and Cys25-His159-Asn175, in papain catalysis: role of Asp158 and Asn175. Protein Eng. 1994 Jan;7(1):75-82. PMID:8140097
- ↑ Ménard R, Khouri HE, Plouffe C, Dupras R, Ripoll D, Vernet T, Tessier DC, Lalberté F, Thomas DY, Storer AC. A protein engineering study of the role of aspartate 158 in the catalytic mechanism of papain. Biochemistry. 1990 Jul 17;29(28):6706-13. PMID:2397208 doi:10.1021/bi00480a021
- ↑ Wang J, Xiang YF, Lim C. The double catalytic triad, Cys25-His159-Asp158 and Cys25-His159-Asn175, in papain catalysis: role of Asp158 and Asn175. Protein Eng. 1994 Jan;7(1):75-82. PMID:8140097
- ↑ [3] The Journal of Biological Chemistry
- ↑ Kamphuis IG, Kalk KH, Swarte MB, Drenth J. Structure of papain refined at 1.65 A resolution. J Mol Biol. 1984 Oct 25;179(2):233-56. PMID:6502713
- ↑ [4] WebMD
- ↑ Kamphuis IG, Kalk KH, Swarte MB, Drenth J. Structure of papain refined at 1.65 A resolution. J Mol Biol. 1984 Oct 25;179(2):233-56. PMID:6502713
- ↑ Kamphuis IG, Kalk KH, Swarte MB, Drenth J. Structure of papain refined at 1.65 A resolution. J Mol Biol. 1984 Oct 25;179(2):233-56. PMID:6502713
- ↑ Tsuge H, Nishimura T, Tada Y, Asao T, Turk D, Turk V, Katunuma N. Inhibition mechanism of cathepsin L-specific inhibitors based on the crystal structure of papain-CLIK148 complex. Biochem Biophys Res Commun. 1999 Dec 20;266(2):411-6. PMID:10600517 doi:10.1006/bbrc.1999.1830
- ↑ [5] University of Maine
- ↑ [6] University of Maine
- ↑ Barretto N, Jukneliene D, Ratia K, Chen Z, Mesecar AD, Baker SC. The papain-like protease of severe acute respiratory syndrome coronavirus has deubiquitinating activity. J Virol. 2005 Dec;79(24):15189-98. PMID:16306590 doi:10.1128/JVI.79.24.15189-15198.2005