Sandbox Reserved 381
From Proteopedia
(→N Terminus) |
(→N Terminus) |
||
| Line 20: | Line 20: | ||
[[Image:Nihms250431f-1-(2).jpg|thumb|200px|right|An overlap of three copies of the TeNT Hc structure. This image helps to illustrate the two separte and distinct domains of the Hc fragment of tetanospasmin.<ref name="hcfrag" />]] | [[Image:Nihms250431f-1-(2).jpg|thumb|200px|right|An overlap of three copies of the TeNT Hc structure. This image helps to illustrate the two separte and distinct domains of the Hc fragment of tetanospasmin.<ref name="hcfrag" />]] | ||
| + | [[Image:File.jpg]] | ||
== O-GlcNAc Modifications == | == O-GlcNAc Modifications == | ||
Revision as of 01:46, 24 November 2011
'Bold text'
| This Sandbox is Reserved from September 14, 2021, through May 31, 2022, for use in the class Introduction to Biochemistry taught by User:John Means at the University of Rio Grande, Rio Grande, OH, USA. This reservation includes 5 reserved sandboxes (Sandbox Reserved 1590 through Sandbox Reserved 1594). |
To get started:
More help: Help:Editing. For an example of a student Proteopedia page, please see Photosystem II, Tetanospasmin, or Guanine riboswitch. |
Contents |
O-GlcNAc transferase
O-linked beta-N-acetylglucosamine transferase (O-GlcNAc transferase) is an essential mammalian enzyme that acts as a nutrient sensor, coupling metabolic status to the regulation of a wide variety of cellular signaling pathways.[1] OGT catalyses the transfer of N-acetylglucosamine from UDP-N-acetylglucosamine (UDP-GlcNAc) to serines and threonines of cytoplasmic, nuclear and mitochondrial proteins, including numerous transcription factors, tumour suppressors, kinases, phospahateses and histone-modifying proteins.[2] Two crystal structures of human OGT are reported here, as a binary complex with UDP (2.8 A resolution) and as a ternary complex with UDP and a peptide substrate (1.95 A).
O-GlcNAc transferase Function
The major mechanism for nutrient sensing in eukaryotes involves OGT. OGt senses cellular glucose levels via UDP-GlcNAc concentration, and responds by O-GlcNAcylating a broad range of nuclear anf cytoplasmic proteins.[3] The N-terminal domain of tetratricopeptide (TPR) mediates the recognition of a broad range of target proteins. Components of the nuclear pore complex are major OGT targets, as OGT depletion by RNA interference (RNAi) results in the loss of GlcNAc modification at the nuclear envelope. The crystal structure of the homodimeric TPR domain of human OGT, which contains 11.5 TPR repeats gives insight into the mechanism of target recognition. The repeats form an elongated superhelix. The concave surface of the superhelix is lined by absolutely conserved asparagines, in a manner reminiscent of the peptide-binding site of importin alpha. Based on this structural similarity, it is proposed that OGT uses an analogous molecular mechanism to recognize its targets.[4]
N Terminus
(Superhelical TPR Domain of O-Linked GlcNAc Transferase)
|
O-GlcNAc Modifications
O-GlcNAc modification has been described for a large and still increasing number of proteins, many of which are key modulators of cellular signalling. O-GlcNAc modifications are catalysed by a OGT, and are removed by the antagonistic enzyme B-N-acetylglucosaminidase (O-GlcNAcase). The general scheme of O-linked N-acetylglucosamine modification suggests that N-acetylglucosamine is added to serine/threonine (Ser/Thr) residues of target proteins by the enzyme OGT using UDP-GlcNac as substrate. The N-acetylglucosamine group is removed by the antagonistic activity of O-GlcNAcase. [6]
Tetanospasmin (TeNT)
Tetanospasmin is a 150-kDa toxin that is composed of one light chain (50-kDa) and one heavy chain (100-kDa). The light chain is responsible for the toxicity of the molecule, whereas the heavy chain is responsible for binding the toxin to the axonal membranes. The heavy chain can also be cleaved into 2 fragments Hn and Hc. The Hn fragment is responsible for the translocation of the light chain across the axonal membrane, whereas the Hc fragment binds to the axonal membrane.[7]
Hc and Ganglioside Interaction
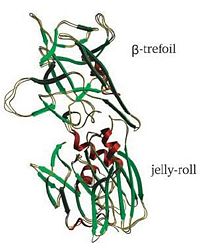
Hc has two distinct domains:[5]
1. Jelly-roll (amino end)
2. β-Trefoil (carboxyl end)
Studies have shown that the β-trefoil domain contains the ganglioside binding sites.[5]
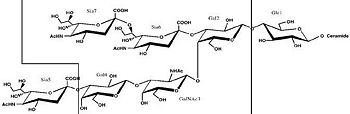
Binding studies have shown that a particular ganglioside, GT1-b, is necessary for the binding of the Hc fragment of tetanospasmin (TeNT). An analogue of the GT1-b ganglioside was made in order to increase solubility because a crystal structure of the Hc and native GT1-b could not be obtained.
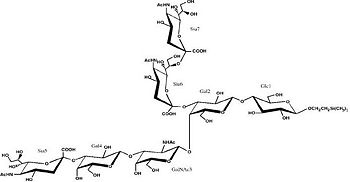
The Hc fragment has two binding sites in the β-trefoil domain:[5]
At this site a narrow groove is formed where a number of hydrogen bonds can form.[5]
Common hydrogen bonds are formed between the side chain of His1271 and OH-6, OH-4 and O-5 of Gal4 and between the main chain carbonyl oxygen of Thr1270 and OH-4 of Gal4. GalNAc3 interacts via a hydrogen bond between OH-4 andAsp1222 OD and between OH-4 and His1271. Ring stacking involving galactose also occurs in this site.
At this site a shallow pocket is formed where hydrogen bonding occurs.[5]
Commonly hydrogen bonds form between OD-1 and OD-2 of Asp1147 and O-4 and the acetamido-N-5 of Sia6 and between ND-2 of Asn1216 and O-10 of Sia6. A salt bridge also forms between Arg1226 and the sialic acid of Sia7, also the carboxylate group and hydrogen bonds between O-1A and the amide NH of Asn1216; between O-4 and the carbonyl oxygen of Asp1214; and betweenOH-8 and Tyr1229 hydroxyl group on Sia7.
Modeling suggests that it is possible for the two arms of the ganglioside to interact with more than one Hc fragment. This may result in clustering and crosslinking of the toxin and enhance the process internalization or uptake of the toxin through the axonal membrane.[5] Others suggest that TeNT can directly cross link two gangliosides through its single protein domain which also enhances the uptake of the toxin.[7] Therefore by binding at one or both of the sites, the Hc fragment of tetanospasmin is successfully able to assist the rest of the tetanospasmin molecule in gaining access to the cytoplasm of the inhibitory interneurons.
References
- ↑ Hart GW, Housley MP, Slawson C. Cycling of O-linked beta-N-acetylglucosamine on nucleocytoplasmic proteins. Nature.2007;446:1017-22.[1]
- ↑ Lazarus MB, Nam Y, Jiang J, Sliz P, Walker S. Structure of human O-GlcNAc transferase and its complex with a peptide substrate. Nature. 2011 Jan 27;469(7331):564-7. Epub 2011 Jan 16. PMID:21240259 doi:10.1038/nature09638
- ↑ Insulin-like signaling pathways and transcriptional activators that regulate glucose levels by controlling gluconeogenisis include proteins that are O-GlcNAcylated by OGT.<ref> Numerous O-GlcNAcylation sites are also phosphorylation sites, OGT is suggested to play a major role in modulating cellular kinase signaling cascades.<ref> Widespread transcriptional regulations also involve OGT.<ref> == O-GlcNAc Structure == OGT is comprised of two distinct regions: a multidomain catalytic region, which has no available structure and an N-terminal region consistin os a seris of tetratricopeptide repeat(TPR)units.<refThe N terminus of OGT is unusual, consisting of 2.5-13.5 tetratricopeptide repeats (TPRs) depending on alternative splicing.<ref>Kreppel L, Hart G. Regulation of a cytosolic and nuclear O-GlcNAc transferase. Role of the tetratricopeptide repeats. J Biol Chem. 1999;274:32015-32022</li> <li id="cite_note-3">[[#cite_ref-3|↑]] Jinek M, Rehwinkel J, Lazarus BD, Izaurralde E, Hanover JA, Conti E. The superhelical TPR-repeat domain of O-linked GlcNAc transferase exhibits structural similarities to importin alpha. Nat Struct Mol Biol. 2004 Oct;11(10):1001-7. Epub 2004 Sep 12. PMID:[http://www.ncbi.nlm.nih.gov/pubmed/15361863 15361863] doi:[http://dx.doi.org/10.1038/nsmb833 10.1038/nsmb833]</li> <li id="cite_note-hcfrag-4">↑ <sup>[[#cite_ref-hcfrag_4-0|5.0]]</sup> <sup>[[#cite_ref-hcfrag_4-1|5.1]]</sup> <sup>[[#cite_ref-hcfrag_4-2|5.2]]</sup> <sup>[[#cite_ref-hcfrag_4-3|5.3]]</sup> <sup>[[#cite_ref-hcfrag_4-4|5.4]]</sup> <sup>[[#cite_ref-hcfrag_4-5|5.5]]</sup> <sup>[[#cite_ref-hcfrag_4-6|5.6]]</sup> <sup>[[#cite_ref-hcfrag_4-7|5.7]]</sup> <sup>[[#cite_ref-hcfrag_4-8|5.8]]</sup> <sup>[[#cite_ref-hcfrag_4-9|5.9]]</sup> Fotinou C, Emsley P, Black I, Ando H, Ishida H, Kiso M, Sinha KA, Fairweather NF, Isaacs NW. The crystal structure of tetanus toxin Hc fragment complexed with a synthetic GT1b analogue suggests cross-linking between ganglioside receptors and the toxin. J Biol Chem. 2001 Aug 24;276(34):32274-81. Epub 2001 Jun 19. PMID:[http://www.ncbi.nlm.nih.gov/pubmed/11418600 11418600] doi:[http://dx.doi.org/10.1074/jbc.M103285200 10.1074/jbc.M103285200]</li> <li id="cite_note-5">[[#cite_ref-5|↑]] Alexander G, Danilo G. The O-linked N-acetylglucosamine modification in cellular signalling and the immune system. EMBO reports. 2008 June;9:748-753[http://www.nature.com/embor/journal/v9/n8/full/embor2008129.html]</li> <li id="cite_note-Affinity-6">↑ <sup>[[#cite_ref-Affinity_6-0|7.0]]</sup> <sup>[[#cite_ref-Affinity_6-1|7.1]]</sup> Chen C, Fu Z, Kim JJ, Barbieri JT, Baldwin MR. Gangliosides as high affinity receptors for tetanus neurotoxin. J Biol Chem. 2009 Sep 25;284(39):26569-77. Epub 2009 Jul 14. PMID:[http://www.ncbi.nlm.nih.gov/pubmed/19602728 19602728] doi:[http://dx.doi.org/10.1074/jbc.M109.027391 10.1074/jbc.M109.027391]</li></ol></ref>
