This old version of Proteopedia is provided for student assignments while the new version is undergoing repairs. Content and edits done in this old version of Proteopedia after March 1, 2026 will eventually be lost when it is retired in about June of 2026.
Apply for new accounts at the new Proteopedia. Your logins will work in both the old and new versions.
Journal:PLoS ONE:2
From Proteopedia
(Difference between revisions)

| Line 9: | Line 9: | ||
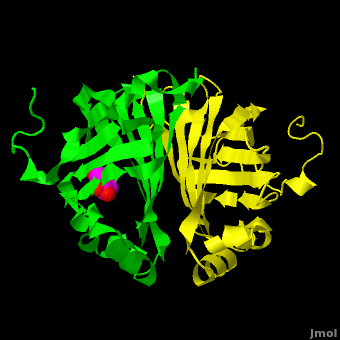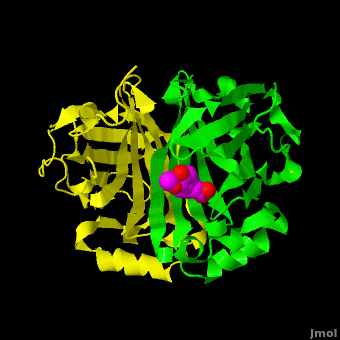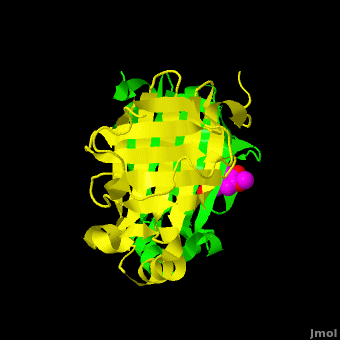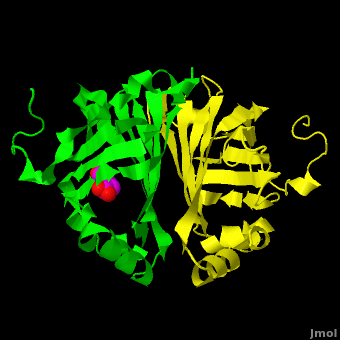
Gu ''et al.'' reported the crystal structure of ferulic acid decarboxylase (FADase) from ''Enterobacter sp.'' Px6-4. The enzyme catalyzes the decarboxylation of ferulic acid, to give 4-vinylguaiacol, a potential precursor for vanillin. The crystals obtained contained two enzyme molecules in the asymmetric unit. One structure was of the “native” conformation, with no substrate bound in the active site of either monomer. <scene name='Journal:PLoS_ONE:2/Cv/4'>A second structure was obtained by soaking the crystals in a sodium ferulate solution.</scene> In this crystal structure <scene name='Journal:PLoS_ONE:2/Cv/6'>one of the enzyme monomers (chain A)</scene> appears to have undergone significant structural changes, with electron density appearing for a <scene name='Journal:PLoS_ONE:2/Cv/7'>small molecule bound in the active site</scene>. The <scene name='Journal:PLoS_ONE:2/Cv/4'>second monomer showed no evidence of change</scene>, and no unusual electron density was observed in the active site. The authors modeled an unreacted ferulate molecule into the density in the active site of the first monomer, and attributed the lack of binding in the second monomer to close crystal contacts, which limit the necessary movements required to allow binding. This latter structure was deposited in the PDB with accession code [[3nx2]]. | Gu ''et al.'' reported the crystal structure of ferulic acid decarboxylase (FADase) from ''Enterobacter sp.'' Px6-4. The enzyme catalyzes the decarboxylation of ferulic acid, to give 4-vinylguaiacol, a potential precursor for vanillin. The crystals obtained contained two enzyme molecules in the asymmetric unit. One structure was of the “native” conformation, with no substrate bound in the active site of either monomer. <scene name='Journal:PLoS_ONE:2/Cv/4'>A second structure was obtained by soaking the crystals in a sodium ferulate solution.</scene> In this crystal structure <scene name='Journal:PLoS_ONE:2/Cv/6'>one of the enzyme monomers (chain A)</scene> appears to have undergone significant structural changes, with electron density appearing for a <scene name='Journal:PLoS_ONE:2/Cv/7'>small molecule bound in the active site</scene>. The <scene name='Journal:PLoS_ONE:2/Cv/4'>second monomer showed no evidence of change</scene>, and no unusual electron density was observed in the active site. The authors modeled an unreacted ferulate molecule into the density in the active site of the first monomer, and attributed the lack of binding in the second monomer to close crystal contacts, which limit the necessary movements required to allow binding. This latter structure was deposited in the PDB with accession code [[3nx2]]. | ||
| - | The editors of the journal were contacted by a reader, who raised concerns about the accuracy of the assignment of the density in the <scene name='Journal:PLoS_ONE:2/Cv/9'>active site of chain A</scene> of [[3nx2]]. The reader suggested that the density was more reminiscent of a <scene name='Journal:PLoS_ONE:2/Cv/10'>HEPES molecule</scene> (used in the crystallization buffer) based on the shape of the density, and the fact that the stereochemistry in the region of the double bond (between atoms C7 and C8) was distorted. <font color='red'><b>O atoms are colored in red</b></font>, <font color='blue'><b>N atoms are in blue</b></font>, <span style="color:yellow;background-color:black;font-weight:bold;">S atom in yellow</span>; <span style="color:cyan;background-color:black;font-weight:bold;">C atoms of HEPES in cyan</span>, and <font color='magenta'><b>C atoms of Ferulate in magenta</b></font>. <scene name='Journal:PLoS_ONE:2/Cv/13'>To see possible superposition of HEPES on Ferulate click here</scene>. In response to these concerns the Editors contacted one of us (JLS), asking for a critical review of the claims of the authors regarding | + | The editors of the journal were contacted by a reader, who raised concerns about the accuracy of the assignment of the density in the <scene name='Journal:PLoS_ONE:2/Cv/9'>active site of chain A</scene> of [[3nx2]]. The reader suggested that the density was more reminiscent of a <scene name='Journal:PLoS_ONE:2/Cv/10'>HEPES molecule</scene> (used in the crystallization buffer) based on the shape of the density, and the fact that the stereochemistry in the region of the double bond (between atoms C7 and C8) was distorted. <font color='red'><b>O atoms are colored in red</b></font>, <font color='blue'><b>N atoms are in blue</b></font>, <span style="color:yellow;background-color:black;font-weight:bold;">S atom in yellow</span>; <span style="color:cyan;background-color:black;font-weight:bold;">C atoms of HEPES in cyan</span>, and <font color='magenta'><b>C atoms of Ferulate in magenta</b></font>. <scene name='Journal:PLoS_ONE:2/Cv/13'>To see possible superposition of HEPES on Ferulate click here</scene>. In response to these concerns the Editors contacted one of us (JLS), asking for a critical review of the claims of the authors regarding [[3nx2]]. The results of this review are detailed below. |
| - | The structure of 3nx2 was downloaded from the PDB as well as the structure factors. The software suite PHENIX[2] was used to run simulated annealing on the structure, with the ligand removed. This produced a simulated annealing Fo - Fc omit map, which should have all structural bias caused by the original ligand removed. A HEPES molecule was fitted into this density using COOT[3], and refined in PHENIX (R = 19.4%, Rfree = 24.4%). Although the R and Rfree are slightly higher than for 3nx2 (R =18.9%, Rfree = 23.6%), this is to be expected, since the geometry of the model refined in PHENIX (rms bonds 0.007, angles 1.12) was much tighter than the one obtained from REFMAC[4] (rms bonds 0.019, angles 1.81). In Figure 2, we superimpose the omit map with the two refined structures: on the left, | + | The structure of [[3nx2]] was downloaded from the PDB as well as the structure factors. The software suite PHENIX[2] was used to run simulated annealing on the structure, with the ligand removed. This produced a simulated annealing Fo - Fc omit map, which should have all structural bias caused by the original ligand removed. A HEPES molecule was fitted into this density using COOT[3], and refined in PHENIX (R = 19.4%, Rfree = 24.4%). Although the R and Rfree are slightly higher than for [[3nx2]] (R =18.9%, Rfree = 23.6%), this is to be expected, since the geometry of the model refined in PHENIX (rms bonds 0.007, angles 1.12) was much tighter than the one obtained from REFMAC[4] (rms bonds 0.019, angles 1.81). In Figure 2, we superimpose the omit map with the two refined structures: on the left, [[3nx2]], with magenta carbon atoms, and on the right, with cyan carbon atoms, the PHENIX-refined enzyme-HEPES complex. |
| - | Although ferulic acid does somewhat match the difference density, the HEPES molecule does appear a better match for the electron density, both in terms of the larger sulfone "head" and the ethoxy tail attached to the ring. While these considerations alone may be insufficient to positively identify the observed density as a HEPES molecule, other considerations make the assignment of this density as ferulate rather difficult. As pointed out by the readers, the carboxylate group of ferulic acid should conjugate with the benzyl system through the double bond between C7 and C8 of ferulic acid. As such, the C8 and the carboxylate group should be coplanar with the benzyl ring. Indeed, in examples of this type of compound in the Cambridge Structural Database (small molecules) these systems are all planar. In the PDB there are 12 submissions (excluding 3NX2) with ferulic acid/ferulate bound. All but two have this system as planar. One of the exceptions is a 1.0Å structure, where there is some deviation of the carboxylate group from planarity, and the other 2wtm, where one of the two molecules of ferulate has been modeled with some deviation from planarity. The authors of 2wtm do not discuss this, and looking at the density maps, this appears to be an oversight on their part, since a planar structure would fit their maps. In the current case of | + | Although ferulic acid does somewhat match the difference density, the HEPES molecule does appear a better match for the electron density, both in terms of the larger sulfone "head" and the ethoxy tail attached to the ring. While these considerations alone may be insufficient to positively identify the observed density as a HEPES molecule, other considerations make the assignment of this density as ferulate rather difficult. As pointed out by the readers, the carboxylate group of ferulic acid should conjugate with the benzyl system through the double bond between C7 and C8 of ferulic acid. As such, the C8 and the carboxylate group should be coplanar with the benzyl ring. Indeed, in examples of this type of compound in the Cambridge Structural Database (small molecules) these systems are all planar. In the PDB there are 12 submissions (excluding 3NX2) with ferulic acid/ferulate bound. All but two have this system as planar. One of the exceptions is a 1.0Å structure, where there is some deviation of the carboxylate group from planarity, and the other 2wtm, where one of the two molecules of ferulate has been modeled with some deviation from planarity. The authors of 2wtm do not discuss this, and looking at the density maps, this appears to be an oversight on their part, since a planar structure would fit their maps. In the current case of [[3nx2]], however, a planar molecule would not fit the density well. |
Furthermore, it is unclear why the authors feel that sodium ferulate would be an inhibitor. As shown by the authors in Figure 6 of Gu et al., the substrate is ferulate. In fact, the authors themselves, in the second paragraph of the Discussion (page 6) refer to the "...enzyme-substrate complex...". Thus, the soaking experiment simply introduced substrate into the crystal, which underwent decarboxylation. Trapping unreacted substrate in the active site would be rather unlikely, and would require supporting evidence to indicate that this has happened. | Furthermore, it is unclear why the authors feel that sodium ferulate would be an inhibitor. As shown by the authors in Figure 6 of Gu et al., the substrate is ferulate. In fact, the authors themselves, in the second paragraph of the Discussion (page 6) refer to the "...enzyme-substrate complex...". Thus, the soaking experiment simply introduced substrate into the crystal, which underwent decarboxylation. Trapping unreacted substrate in the active site would be rather unlikely, and would require supporting evidence to indicate that this has happened. | ||
| - | In light of the above, we propose the following explanation for the observations in the native structure (3NX1), and in the complex ( | + | In light of the above, we propose the following explanation for the observations in the native structure (3NX1), and in the complex ([[3nx2]]). |
1. HEPES is a relatively weak competitive inhibitor of FADase. In the native crystals, the binding constant of HEPES was not large enough to effect the conformational change necessary to allow binding in the crystal structure, despite the fact that HEPES was present in the crystallization mixture. | 1. HEPES is a relatively weak competitive inhibitor of FADase. In the native crystals, the binding constant of HEPES was not large enough to effect the conformational change necessary to allow binding in the crystal structure, despite the fact that HEPES was present in the crystallization mixture. | ||
Revision as of 13:37, 24 November 2011
| |||||||||||
- ↑ Gu W, Yang J, Lou Z, Liang L, Sun Y, Huang J, Li X, Cao Y, Meng Z, Zhang KQ. Structural Basis of Enzymatic Activity for the Ferulic Acid Decarboxylase (FADase) from Enterobacter sp. Px6-4. PLoS One. 2011 Jan 21;6(1):e16262. PMID:21283705 doi:10.1371/journal.pone.0016262
This page complements a publication in scientific journals and is one of the Proteopedia's Interactive 3D Complement pages. For aditional details please see I3DC.