User:Gourinchas Geoffrey/Sandbox 205
From Proteopedia
(Difference between revisions)
| Line 27: | Line 27: | ||
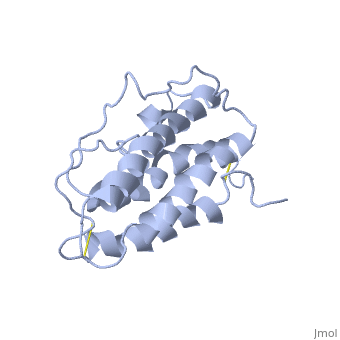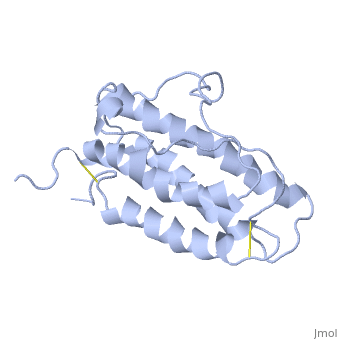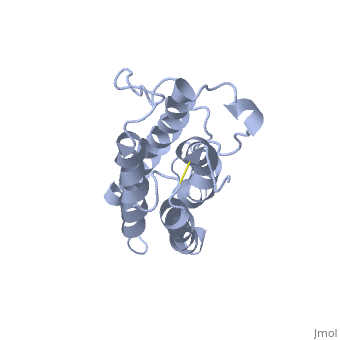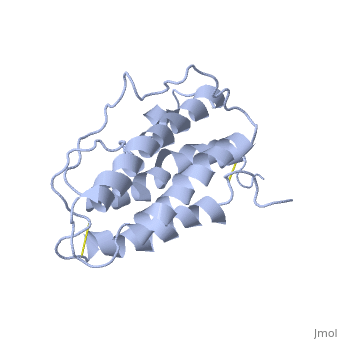
The molecule of Erythropoietin have two opposite binding sites with his receptor. The first site include segments of αA, αB and αD and a part of the loop which connects αA and αB. This site include a hydrophobic center which interact with the receptor. | The molecule of Erythropoietin have two opposite binding sites with his receptor. The first site include segments of αA, αB and αD and a part of the loop which connects αA and αB. This site include a hydrophobic center which interact with the receptor. | ||
| - | The Phenylalanine 93 of the receptor is critical important for binding of Erythropoietin to his receptor thanks to hydrogen bonds with residues <scene name='User:Gourinchas_Geoffrey/Sandbox_205/Threonine_44_n2/1'>Threonine 44</scene> and <scene name='User:Gourinchas_Geoffrey/Sandbox_205/Asparagine_147/2'>Asparagine 147</scene> of Erythropoietin. </StructureSection> | + | The Phenylalanine 93 of the receptor is critical important for binding of Erythropoietin to his receptor thanks to hydrogen bonds with residues <scene name='User:Gourinchas_Geoffrey/Sandbox_205/Threonine_44_n2/1'>Threonine 44</scene> and <scene name='User:Gourinchas_Geoffrey/Sandbox_205/Asparagine_147/2'>Asparagine 147</scene> of Erythropoietin. |
| + | |||
| + | In the second interaction site the Methionine 150 in second site allows van der waals with Arginine 10, the Valine 11 and the Arginine 14 of Erythropoietin. | ||
| + | |||
| + | The hydrophobic interaction include 11 hydrogen bonds between αA and αC of Erythropoietin and his receptor. </StructureSection> | ||
Revision as of 22:15, 27 November 2011
HUMAN ERYTHROPOIETIN
|
Erythropoietin is a glycoprotein hormone which is involved in Erythropoiesis, which is the red blood cells production. It allows the differenciation of the erythrocyte precursors in the bone marrow.
Structure
| |||||||||||