User:Brian Hernandez/DOPA Decarboxylase
From Proteopedia
| Line 7: | Line 7: | ||
==Structure== | ==Structure== | ||
---- | ---- | ||
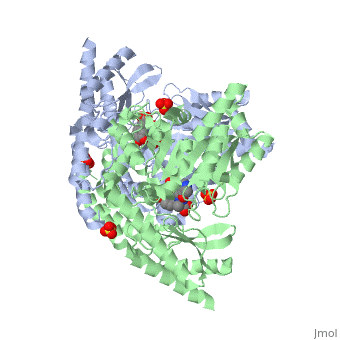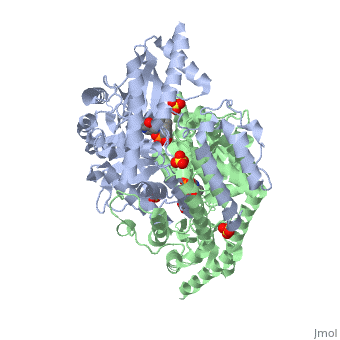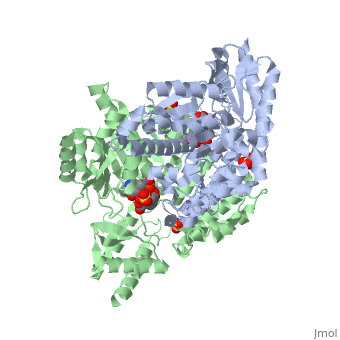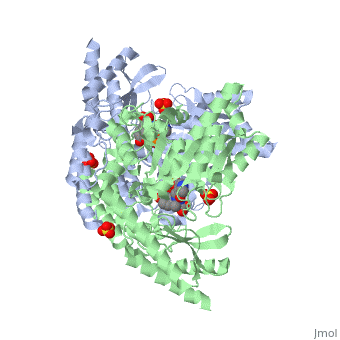
| - | DDC consists of two monomers [SC], each with three distinct domains: the large domain [SC], the C-terminal small domain [SC], and the N-terminal domain [SC]. The <scene name='DOPA_decarboxylase/Large_domain/1'>large domain</scene> consists of the cofactor (PLP- pyridoxal phosphate) binding site and is comprised of a central, seven-stranded mixed β-sheet surrounded by eight α-helices [SC] in a typical α/β fold. The small <scene name='DOPA_decarboxylase/Small_domain/1'>C-terminal domain</scene> consists of a four-stranded antiparallel β-sheet with three helices[SC] packed against the face opposite the large domain. The N-terminal domain (characteristic of all α-family enzymes) is composed of two parallel helices linked by an extended strand [SC]. This structure flaps over the top of the second subunit and vice versa, with the first helix of one subunit aligning parallel to the equivalent helix of the other subunit, thereby forming the extended dimer interface. Although it is unlikely that this domain represents an autonomous folding unit, it is most likely stable only in the context of the dimer by extending the interface between the two monomers. | + | DDC consists of two monomers [SC], each with three distinct domains: the large domain [SC], the C-terminal small domain [SC], and the N-terminal domain [SC]. The <scene name='DOPA_decarboxylase/Large_domain/1'>large domain</scene> consists of the cofactor (PLP- pyridoxal phosphate) binding site and is comprised of a central, seven-stranded mixed β-sheet surrounded by eight α-helices [SC] in a typical α/β fold. The small <scene name='DOPA_decarboxylase/Small_domain/1'>C-terminal domain</scene> consists of a four-stranded antiparallel β-sheet with three helices[SC] packed against the face opposite the large domain. The <scene name='Sandbox/N-terminal_domain/2'>N-terminal domain</scene> (characteristic of all α-family enzymes) is composed of two parallel helices linked by an extended strand [SC]. This structure flaps over the top of the second subunit and vice versa, with the first helix of one subunit aligning parallel to the equivalent helix of the other subunit, thereby forming the extended dimer interface. Although it is unlikely that this domain represents an autonomous folding unit, it is most likely stable only in the context of the dimer by extending the interface between the two monomers. |
==Function== | ==Function== | ||
Revision as of 04:36, 29 November 2011
| |||||||||||
DDC and Parkinson's Disease
Treatment
Parkinson's disease, a neurological disorder, can be characterized by tremor, bradykinesia, rigidity, and postural instability. With it's possible relation to degenerative dopamine-producing cells in the brain, administration of L-DOPA can increase the amount of synthesized dopamine in the nerve cell; direct treatment with dopamine is not sufficient as dopamine itself cannot pass the blood-brain barrier. However, only a small percentage of the dose actually reaches the nervous system, with the remaining majority being rapidly converted to dopamine in the blood stream. This dopamine-rich blood causes side effects of nausea, daytime sleepiness, orthostatic hypotension, involuntary movements, decreased appetite, insomnia, and cramping. Addition of a DDC inhibitor would block peripheral conversion to dopamine and allow a greater percentage of L-DOPA to reach the brain, causing an increase in brain dopamine levels, and diminishing the side effects of dopamine-rich blood.