1osm
From Proteopedia
m (Protected "1osm" [edit=sysop:move=sysop]) |
|||
| Line 1: | Line 1: | ||
[[Image:1osm.png|left|200px]] | [[Image:1osm.png|left|200px]] | ||
| - | <!-- | ||
| - | The line below this paragraph, containing "STRUCTURE_1osm", creates the "Structure Box" on the page. | ||
| - | You may change the PDB parameter (which sets the PDB file loaded into the applet) | ||
| - | or the SCENE parameter (which sets the initial scene displayed when the page is loaded), | ||
| - | or leave the SCENE parameter empty for the default display. | ||
| - | --> | ||
{{STRUCTURE_1osm| PDB=1osm | SCENE= }} | {{STRUCTURE_1osm| PDB=1osm | SCENE= }} | ||
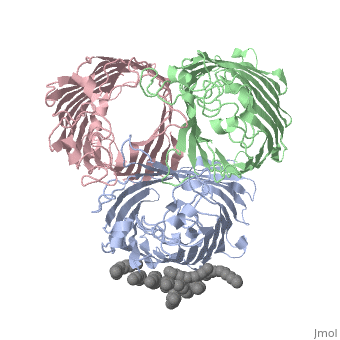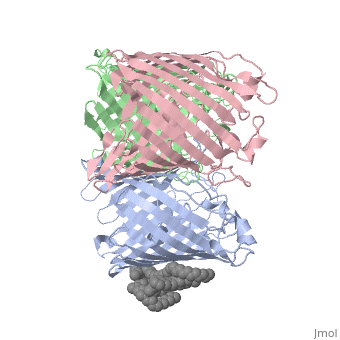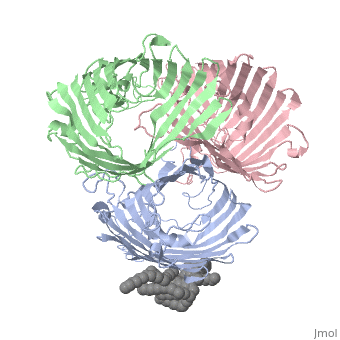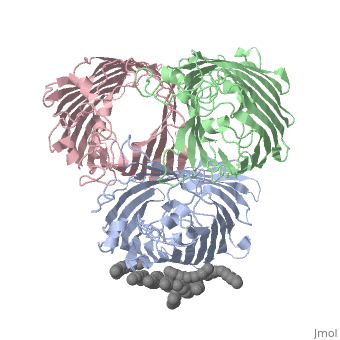
===OSMOPORIN (OMPK36) FROM KLEBSIELLA PNEUMONIAE=== | ===OSMOPORIN (OMPK36) FROM KLEBSIELLA PNEUMONIAE=== | ||
| - | |||
| - | <!-- | ||
| - | The line below this paragraph, {{ABSTRACT_PUBMED_10196126}}, adds the Publication Abstract to the page | ||
| - | (as it appears on PubMed at http://www.pubmed.gov), where 10196126 is the PubMed ID number. | ||
| - | --> | ||
{{ABSTRACT_PUBMED_10196126}} | {{ABSTRACT_PUBMED_10196126}} | ||
| Line 22: | Line 11: | ||
==See Also== | ==See Also== | ||
| - | *[[Porin]] | + | *[[Porin|Porin]] |
==Reference== | ==Reference== | ||
Revision as of 12:45, 26 July 2012
| |||||||||
| 1osm, resolution 3.20Å () | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Ligands: | |||||||||
| |||||||||
| |||||||||
| Resources: | FirstGlance, OCA, RCSB, PDBsum | ||||||||
| Coordinates: | save as pdb, mmCIF, xml | ||||||||
Contents |
OSMOPORIN (OMPK36) FROM KLEBSIELLA PNEUMONIAE
BACKGROUND: Porins are channel-forming membrane proteins that confer solute permeability to the outer membrane of Gram-negative bacteria. In Escherichia coli, major nonspecific porins are matrix porin (OmpF) and osmoporin (OmpC), which show high sequence homology. In response to high osmolarity of the medium, OmpC is expressed at the expense of OmpF porin. Here, we study osmoporin of the pathogenic Klebsiella pneumoniae (OmpK36), which shares 87% sequence identity with E. coliOmpC in an attempt to establish why osmoporin is best suited to function at high osmotic pressure. RESULTS: The crystal structure of OmpK36 has been determined to a resolution of 3.2 A by molecular replacement with the model of OmpF. The structure of OmpK36 closely resembles that of the search model. The homotrimeric structure is composed of three hollow 16-stranded antiparallel beta barrels, each delimiting a separate pore. Most insertions and deletions with respect to OmpF are found in the loops that protrude towards the cell exterior. A characteristic ten-residue insertion in loop 4 contributes to the subunit interface. At the pore constriction, the replacement of an alanine by a tyrosine residue does not alter the pore profile of OmpK36 in comparison with OmpF because of the different course of the mainchain. Functionally, as characterized in lipid bilayers and liposomes, OmpK36 resembles OmpC with decreased conductance and increased cation selectivity in comparison with OmpF. CONCLUSIONS: The osmoporin structure suggests that not an altered pore size but an increase in charge density is the basis for the distinct physico-chemical properties of this porin that are relevant for its preferential expression at high osmotic strength.
Crystal structure and functional characterization of OmpK36, the osmoporin of Klebsiella pneumoniae., Dutzler R, Rummel G, Alberti S, Hernandez-Alles S, Phale P, Rosenbusch J, Benedi V, Schirmer T, Structure. 1999 Apr 15;7(4):425-34. PMID:10196126
From MEDLINE®/PubMed®, a database of the U.S. National Library of Medicine.
About this Structure
1osm is a 3 chain structure of Porin with sequence from Klebsiella pneumoniae. Full crystallographic information is available from OCA.
See Also
Reference
- Dutzler R, Rummel G, Alberti S, Hernandez-Alles S, Phale P, Rosenbusch J, Benedi V, Schirmer T. Crystal structure and functional characterization of OmpK36, the osmoporin of Klebsiella pneumoniae. Structure. 1999 Apr 15;7(4):425-34. PMID:10196126