FK506 binding protein
From Proteopedia
| Line 27: | Line 27: | ||
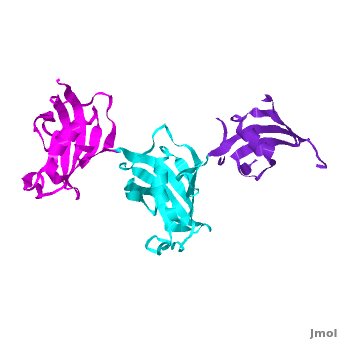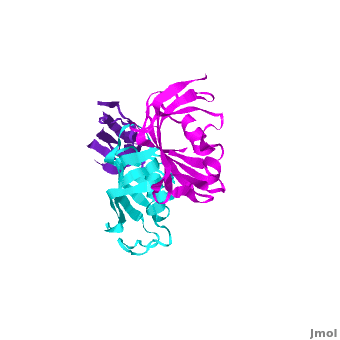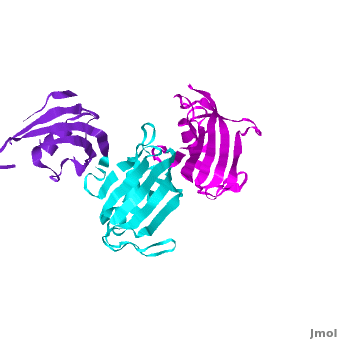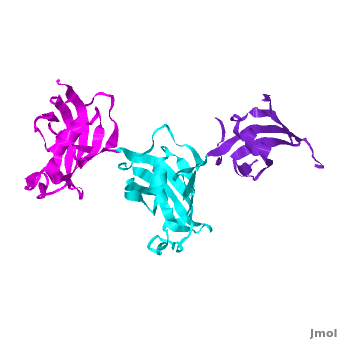
It was shown that 12 conserved residues (for wFK73_1 domain they are Tyr67, Phe77, Asp78, Arg83, Phe87, Gln95, Val96, Ile97, Trp100, Tyr123, Ile132, and Phe140) of the FK1 domains of hFKBP12, 13, 25, 51 and 52, are involved in binding the FK506 or rapamycin. Since only the FK1 domains contain all the conserved amino acids (in contrast to FK2 and/or FK3 domains), only they exhibit PPIase activity, which can be inhibited by the binding of the drugs FK506, and rapamycin. These conserved residues form the hydrophobic cavity. The structure of hFKBP12 ([[2ppn]]) demonstrates a good example of this <scene name='3jym/Cavity/1'>cavity</scene>. All these residues are conserved in the wFK73_1 domain, it could be assumed that a similar cavity is also formed in wFK73_1, although some of these residues are missing electron density in the wFK73 structure and, therefore, it can not be seen. Domain <font color='magenta'><b>wFK73_3</b></font> has <scene name='3jym/Cavity/3'>narrower cavity</scene>, whereas <font color='cyan'><b>wFK73_2</b></font> <scene name='3jym/Cavity/4'>lacks this cavity at all</scene>. Conserved residues are colored yellow. So, the lack of drug binding of the wFK73_2 and wFK73_3 domains could be explained by the absence of the conserved drug binding residues. This is in agreement with the fact that the FK2 domains of hFKBP51 and hFKBP52 and the single FK domains of FKBP38, DmFKBP45 and AtFKBP42, all lacking the conserved residues, do not exhibit drug binding. | It was shown that 12 conserved residues (for wFK73_1 domain they are Tyr67, Phe77, Asp78, Arg83, Phe87, Gln95, Val96, Ile97, Trp100, Tyr123, Ile132, and Phe140) of the FK1 domains of hFKBP12, 13, 25, 51 and 52, are involved in binding the FK506 or rapamycin. Since only the FK1 domains contain all the conserved amino acids (in contrast to FK2 and/or FK3 domains), only they exhibit PPIase activity, which can be inhibited by the binding of the drugs FK506, and rapamycin. These conserved residues form the hydrophobic cavity. The structure of hFKBP12 ([[2ppn]]) demonstrates a good example of this <scene name='3jym/Cavity/1'>cavity</scene>. All these residues are conserved in the wFK73_1 domain, it could be assumed that a similar cavity is also formed in wFK73_1, although some of these residues are missing electron density in the wFK73 structure and, therefore, it can not be seen. Domain <font color='magenta'><b>wFK73_3</b></font> has <scene name='3jym/Cavity/3'>narrower cavity</scene>, whereas <font color='cyan'><b>wFK73_2</b></font> <scene name='3jym/Cavity/4'>lacks this cavity at all</scene>. Conserved residues are colored yellow. So, the lack of drug binding of the wFK73_2 and wFK73_3 domains could be explained by the absence of the conserved drug binding residues. This is in agreement with the fact that the FK2 domains of hFKBP51 and hFKBP52 and the single FK domains of FKBP38, DmFKBP45 and AtFKBP42, all lacking the conserved residues, do not exhibit drug binding. | ||
| + | </StructureSection> | ||
| + | ==SlyD == | ||
| + | <StructureSection load='2kr7' size='500' side='right' scene='Journal:JBIC:14/Cv/2' caption=''> | ||
| + | === Multifaceted SlyD from ''Helicobacter pylori'': implication in [NiFe] hydrogenase maturation === | ||
| + | <big>Tianfan Cheng, Hongyan Li, Wei Xia and Hongzhe Sun</big><ref >DOI 10.1007/s00775-011-0855-y</ref> | ||
| + | <hr/> | ||
| + | <b>Molecular Tour</b><br> | ||
| + | SlyD belongs to the FK506-binding protein (FKBP) family with both peptidylprolyl isomerase (PPIase) and chaperone activities, and is considered to be a ubiquitous cytosolic protein-folding facilitator in bacteria. It possesses a histidine- and cysteine-rich C-terminus binding to selected divalent metal ions (''e.g.'', Ni<sup>2+</sup>, Zn<sup>2+</sup>), which is important for its involvement in the maturation processes of metalloenzymes. We have determined the solution structure of <scene name='Journal:JBIC:14/Cv/3'>C-terminus-truncated SlyD</scene> from ''Helicobacter pylori'' (HpSlyDΔC). HpSlyDΔC folds into <scene name='Journal:JBIC:14/Cv/4'>two well-separated, orientation-independent domains:</scene> the <span style="color:cyan;background-color:black;font-weight:bold;">PPIase-active FKBP domain (in cyan)</span> and the <font color='red'><b>chaperone-active insert-in-flap (IF) domain (in red)</b></font>, <font color='darkmagenta'><b>linkers are in darkmagenta</b></font>. The FKBP domain consists of a four-stranded antiparallel <scene name='Journal:JBIC:14/Cv/5'>β-sheet with an α-helix on one side, whereas the IF domain folds into a four-stranded antiparallel β-sheet accompanied by a short α-helix.</scene> Intact ''H. pylori'' SlyD binds both Ni<sup>2+</sup> and Zn<sup>2+</sup>, with dissociation constants of 2.74 and 3.79 μM respectively. Intriguingly, binding of Ni<sup>2+</sup> instead of Zn<sup>2+</sup> induces protein conformational changes around the <scene name='Journal:JBIC:14/Cv/6'>active sites of the FKBP domain, implicating a regulatory role of nickel</scene> <font color='blueviolet'><b>(residues experiencing relatively large chemical shift perturbations upon interactions of HpSlyDΔC with Ni<sup>2+</sup> are in blueviolet)</b></font>. <scene name='Journal:JBIC:14/Cv/7'>The twin-arginine translocation (Tat) signal peptide from the small subunit of [NiFe] hydrogenase (HydA) binds the protein at the IF domain</scene> <font color='orange'><b>(residues in orange)</b></font>. Surprisingly, several residues (Ile41, Gly42, Ile46, and Asn31) were from the FKBP domain, which is likely due to the binding of the longer n-region of HydA Tat peptide to the FKBP domain. Nickel binding and the recognition of the Tat signal peptide by the protein suggest that SlyD participates in [NiFe] hydrogenase maturation processes. | ||
| + | |||
</StructureSection> | </StructureSection> | ||
Revision as of 10:51, 8 December 2011
FK506 binding protein (FKBP) is a prolyl isomerase related to the cyclophilins. FKBP is a folding chaperone for proteins containing prolines. FKBP12 binds the immunosuppressor tacrolimus (FK506) which is used against organ rejection.
Contents |
Wheat FKBP73
| |||||||||||
SlyD
| |||||||||||
3D Structures of FKBP
FKPB3
3kz7 – hFKBP FK506-binding domain + immunosuppressant - human
FKPB4
1q1c – hFKBP
1n1a – hFKBP N terminal
1p5q - hFKBP C terminal
1qz2 – hFKBP + Hsp90 peptide
FKBP5
3o5d, 3o5e, 3o5f – hFKBP
3o5g, 3o5i, 3o5j, 3o5k - hFKBP FK506-binding domain
3o5l, 3o5m, 3o5o, 3o5p, 3p5q - hFKBP FK506-binding domain (mutant)
3o5r - hFKBP FK506-binding domain (mutant) + immunosuppressant
FKBP8
2f2d, 3ey6, 2awg - hFKBP FK506-binding domain
2d9f – hFKBP – NMR
2jwx - hFKBP N terminal - NMR
FKBP12
1eym – hFKBP (mutant)
1fkk – hFKBP
2gaq, 2pnu– hFKBP - NMR
1fkd, 1fkj, 2fke, 1qpf, 1qpl – hFKBP + immunosuppressant
2ppp, 2ppn, 2dg3, 1d6o – hFKBP
1j4h, 1j4i – hFKBP + inhibitor
1b6c – hFKBP + TGF-B superfamily receptor I
3fap – hFKBP + FKBP12-rapamycin associated protein
4fap - hFKBP + FKBP12-rapamycin associated protein + immunosuppressant
1tco - FKBP + Ser/Thr phosphatase B2 + immunosuppressant - bovine
1yat – FKBP + antagonist – yeast
FKBP26
3pr9, 3pra, 3prb, 3prd – FKBP – Methanocaldococcus jannaschii
FKBP59
1rot, 1rou – FKBP N terminal – NMR – rabbit
2kr7 – FKBP SlyD – NMR - Helicobacter pylori
2lgo – FKBP – NMR – Giardia lamblia
FKBP73
3jym - FKBP wheat