We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
Journal:PLoS ONE:2
From Proteopedia
(Difference between revisions)

| Line 15: | Line 15: | ||
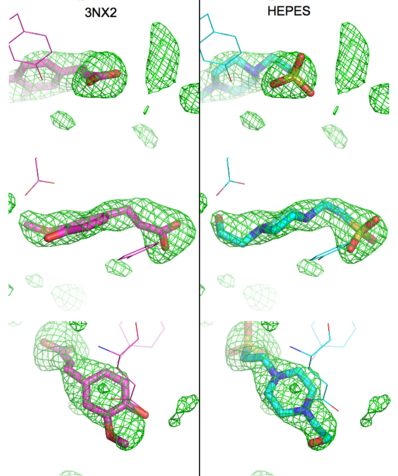
[[Image:Elden1.png|left|400px|thumb|'''Figure:''' Simulated annealing omit map (Fo – Fc) in region of active site, contoured at 3σ. On the left is the superposition of the structure of ferulate from [[3nx2]] (<font color='magenta'><b>magenta C atoms</b></font>), and on the right the refined HEPES molecule (<span style="color:cyan;background-color:black;font-weight:bold;">cyan C atoms</span>). The three views show different aspects of the density and modeled molecules. In addition we have included Tyr27 and Glu134 from each respective structure. Prepared with [http://www.pymol.org PyMOL].]] | [[Image:Elden1.png|left|400px|thumb|'''Figure:''' Simulated annealing omit map (Fo – Fc) in region of active site, contoured at 3σ. On the left is the superposition of the structure of ferulate from [[3nx2]] (<font color='magenta'><b>magenta C atoms</b></font>), and on the right the refined HEPES molecule (<span style="color:cyan;background-color:black;font-weight:bold;">cyan C atoms</span>). The three views show different aspects of the density and modeled molecules. In addition we have included Tyr27 and Glu134 from each respective structure. Prepared with [http://www.pymol.org PyMOL].]] | ||
{{Clear}} | {{Clear}} | ||
| - | Although ferulic acid does somewhat match the difference density, the HEPES molecule does appear a better match for the electron density, both in terms of the larger sulfone "head" and the ethoxy tail attached to the ring. While these considerations alone may be insufficient to positively identify the observed density as a HEPES molecule, other considerations make the assignment of this density as ferulate rather difficult. As pointed out by the readers, the <scene name='Journal:PLoS_ONE:2/Cv4/1'>carboxylate group</scene> of ferulic acid should conjugate with the <scene name='Journal:PLoS_ONE:2/Cv4/3'>benzyl system</scene>through the <scene name='Journal:PLoS_ONE:2/Cv4/4'>double bond between C7 and C8</scene> of ferulic acid. As such, the C8 and the carboxylate group should be coplanar with the benzyl ring. Indeed, in examples of this type of compound in the Cambridge Structural Database (small molecules) these systems are all planar. In the PDB there are 12 submissions (excluding [[3nx2]]) with ferulic acid/ferulate bound. All but two have this system as planar. One of the exceptions is a 1.0Å structure, where there is some deviation of the carboxylate group from planarity, and the other [[2wtm]], where one of the two molecules of ferulate has been modeled with some deviation from planarity. The authors of [[2wtm]] do not discuss this, and looking at the density maps, this appears to be an oversight on their part, since a planar structure would fit their maps. In the current case of [[3nx2]], however, a planar molecule would not fit the density well. | + | Although ferulic acid does somewhat match the difference density, the HEPES molecule does appear a better match for the electron density, both in terms of the larger sulfone "head" and the ethoxy tail attached to the ring. While these considerations alone may be insufficient to positively identify the observed density as a HEPES molecule, other considerations make the assignment of this density as ferulate rather difficult. As pointed out by the readers, the <scene name='Journal:PLoS_ONE:2/Cv4/1'>carboxylate group</scene> of ferulic acid should conjugate with the <scene name='Journal:PLoS_ONE:2/Cv4/3'>benzyl system</scene>through the <scene name='Journal:PLoS_ONE:2/Cv4/4'>double bond between C7 and C8</scene> of ferulic acid. As such, the <scene name='Journal:PLoS_ONE:2/Cv4/5'>C8 and the carboxylate group should be coplanar with the benzyl ring</scene>. Indeed, in examples of this type of compound in the Cambridge Structural Database (small molecules) these systems are all planar. In the PDB there are 12 submissions (excluding [[3nx2]]) with ferulic acid/ferulate bound. All but two have this system as planar. One of the exceptions is a 1.0Å structure, where there is some deviation of the carboxylate group from planarity, and the other [[2wtm]], where one of the two molecules of ferulate has been modeled with some deviation from planarity. The authors of [[2wtm]] do not discuss this, and looking at the density maps, this appears to be an oversight on their part, since a planar structure would fit their maps. In the current case of [[3nx2]], however, a planar molecule would not fit the density well. |
Furthermore, it is unclear why the authors feel that sodium ferulate would be an inhibitor. As shown by the authors in Figure 6 of Gu ''et al.'' <ref name="Gu"/>, the substrate is ferulate. In fact, the authors themselves, in the second paragraph of the Discussion (page 6) refer to the "...enzyme-substrate complex...". Thus, the soaking experiment simply introduced substrate into the crystal, which underwent decarboxylation. Trapping unreacted substrate in the active site would be rather unlikely, and would require supporting evidence to indicate that this has happened. | Furthermore, it is unclear why the authors feel that sodium ferulate would be an inhibitor. As shown by the authors in Figure 6 of Gu ''et al.'' <ref name="Gu"/>, the substrate is ferulate. In fact, the authors themselves, in the second paragraph of the Discussion (page 6) refer to the "...enzyme-substrate complex...". Thus, the soaking experiment simply introduced substrate into the crystal, which underwent decarboxylation. Trapping unreacted substrate in the active site would be rather unlikely, and would require supporting evidence to indicate that this has happened. | ||
Revision as of 12:53, 11 December 2011
| |||||||||||
- ↑ 1.0 1.1 1.2 Gu W, Yang J, Lou Z, Liang L, Sun Y, Huang J, Li X, Cao Y, Meng Z, Zhang KQ. Structural Basis of Enzymatic Activity for the Ferulic Acid Decarboxylase (FADase) from Enterobacter sp. Px6-4. PLoS One. 2011 Jan 21;6(1):e16262. PMID:21283705 doi:10.1371/journal.pone.0016262
- ↑ Adams PD, Afonine PV, Bunkoczi G, Chen VB, Davis IW, Echols N, Headd JJ, Hung LW, Kapral GJ, Grosse-Kunstleve RW, McCoy AJ, Moriarty NW, Oeffner R, Read RJ, Richardson DC, Richardson JS, Terwilliger TC, Zwart PH. PHENIX: a comprehensive Python-based system for macromolecular structure solution. Acta Crystallogr D Biol Crystallogr. 2010 Feb;66(Pt 2):213-21. Epub 2010, Jan 22. PMID:20124702 doi:10.1107/S0907444909052925
- ↑ Emsley P, Lohkamp B, Scott WG, Cowtan K. Features and development of Coot. Acta Crystallogr D Biol Crystallogr. 2010 Apr;66(Pt 4):486-501. Epub 2010, Mar 24. PMID:20383002 doi:10.1107/S0907444910007493
- ↑ Murshudov GN, Vagin AA, Dodson EJ. Refinement of macromolecular structures by the maximum-likelihood method. Acta Crystallogr D Biol Crystallogr. 1997 May 1;53(Pt 3):240-55. PMID:15299926 doi:http://dx.doi.org/10.1107/S0907444996012255
This page complements a publication in scientific journals and is one of the Proteopedia's Interactive 3D Complement pages. For aditional details please see I3DC.