User:Gourinchas Geoffrey/Sandbox 205
From Proteopedia
| Line 44: | Line 44: | ||
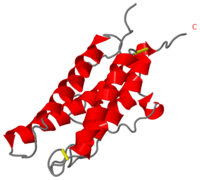
It is composed to 4 alpha-helix: αA, αB, αC and αD which are associated the some in front of the others. | It is composed to 4 alpha-helix: αA, αB, αC and αD which are associated the some in front of the others. | ||
| - | αA et αD are held together by a disulfide bond between <scene name='User:Gourinchas_Geoffrey/Sandbox_205/ | + | αA et αD are held together by a disulfide bond between <scene name='User:Gourinchas_Geoffrey/Sandbox_205/Cysteine_7_n3/1'>Cysteine 7</scene> and <scene name='User:Gourinchas_Geoffrey/Sandbox_205/Cysteine_161_n3/1'>Cysteine 161</scene>. |
αB et αC are held together by a short loop. | αB et αC are held together by a short loop. | ||
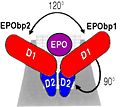
The molecule of Erythropoietin have two opposite binding sites with his receptor. The first site include segments of αA, αB and αD and a part of the loop which connects αA and αB. This site include a hydrophobic center which interact with the receptor. | The molecule of Erythropoietin have two opposite binding sites with his receptor. The first site include segments of αA, αB and αD and a part of the loop which connects αA and αB. This site include a hydrophobic center which interact with the receptor. | ||
| - | The Phenylalanine 93 of the receptor is critical important for binding of Erythropoietin to his receptor thanks to hydrogen bonds with residues <scene name='User:Gourinchas_Geoffrey/Sandbox_205/ | + | The Phenylalanine 93 of the receptor is critical important for binding of Erythropoietin to his receptor thanks to hydrogen bonds with residues <scene name='User:Gourinchas_Geoffrey/Sandbox_205/Threonine_44_n3/1'>Threonine 44</scene> and <scene name='User:Gourinchas_Geoffrey/Sandbox_205/Asparagine_147/3'>Asparagine 147</scene> of Erythropoietin. |
In the second interaction site, the Methionine 150 allows van der waals interactions with Arginine 10, the Valine 11 and the Arginine 14 of Erythropoietin. | In the second interaction site, the Methionine 150 allows van der waals interactions with Arginine 10, the Valine 11 and the Arginine 14 of Erythropoietin. | ||
| Line 62: | Line 62: | ||
The intracellular surface create by ths angle of 120° allows the optimal induction of Erythropoietin by the intracellulaire way of Kinase. </StructureSection> | The intracellular surface create by ths angle of 120° allows the optimal induction of Erythropoietin by the intracellulaire way of Kinase. </StructureSection> | ||
αα | αα | ||
| + | |||
| + | |||
| + | == Application in medicine == | ||
Revision as of 16:20, 22 December 2011
Contents |
HUMAN ERYTHROPOIETIN
|
The Erythropoietin hormone is a glycoprotein which is involved in Erythropoiesis, which is the red blood cells production. It allows the differenciation of the erythrocyte precursors in the bone marrow.
Biological role
L'Erythropoietin is a glycosylated hormone mainly secreted in the kidneys (90%) and little in the liver. The secretion of this hormone depend of the partial pressure of dioxygen in the kidney cells. Indeed, if there is a diminution of the dioxygen pressure, the number of red blood cells decreases, for example when your are at high altitude, or if you are a big hemorrhagic wound. Thus needs of dioxygen of the tissues increase and thus the secretion of Erythropoietin is stimulated.
On the contrary, an increase of the pressure of dioxygen decrease the secretion of Erythropoietin.
To increase the number of red blood cells, this hormone stimulate the proliferation of stem cells precursor of erythrocyte in the bone marrow which will became red blood cells. The glycosylation of this hormone allows his solubilisation in the blood and his carry towards his target, the bone marrow.
| |||||||||||
αα