User:Gourinchas Geoffrey/Sandbox 205
From Proteopedia
| Line 53: | Line 53: | ||
of Erythropoietin, J. Mol. Biol. (2011) 412, 536–550</ref>: indeed if the molecule loses its acids sialiques, stripped galactoses are recognized by the specific hepatic receptor, also the molecule is evacuated then quickly by the circulation. On the contrary,if the Erythropoietin is completely no glycosylated, it is going to aggregated and to become completely inactive in vivo. | of Erythropoietin, J. Mol. Biol. (2011) 412, 536–550</ref>: indeed if the molecule loses its acids sialiques, stripped galactoses are recognized by the specific hepatic receptor, also the molecule is evacuated then quickly by the circulation. On the contrary,if the Erythropoietin is completely no glycosylated, it is going to aggregated and to become completely inactive in vivo. | ||
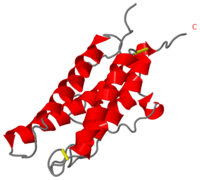
| - | The tertiary structure of this hormone is composed to 4 alpha-helix: αA, αB, αC and αD which are associated the some in front of the others. | + | The tertiary structure <ref>Por-Hsiung Lai, RicharEd verett, Fung-Fang Wang, Tsutomu Arakawa, and Eugene Goldwas, Structural Characterization of Human Erythropoietin, THE JOURNAL OF BIOLOGICAL CHEMISTRY (1986) vol.261 N°7 3116-3121</ref> <ref>Boissel JP, Lee WR, Presnell SR, Cohen FE, Bunn HF. Erythropoietin structure-function relationships. Mutant proteins that test a model of tertiary structure. J Biol Chem. 1993 Jul 25;268(21):15983-93. PubMed PMID:8340419</ref> of this hormone is composed to 4 alpha-helix: αA, αB, αC and αD which are associated the some in front of the others. |
αA et αD are held together by a disulfide bond between <scene name='User:Gourinchas_Geoffrey/Sandbox_205/Cysteine_7_n3/1'>Cysteine 7</scene> and <scene name='User:Gourinchas_Geoffrey/Sandbox_205/Cysteine_161_n3/1'>Cysteine 161</scene>. | αA et αD are held together by a disulfide bond between <scene name='User:Gourinchas_Geoffrey/Sandbox_205/Cysteine_7_n3/1'>Cysteine 7</scene> and <scene name='User:Gourinchas_Geoffrey/Sandbox_205/Cysteine_161_n3/1'>Cysteine 161</scene>. | ||
| Line 64: | Line 64: | ||
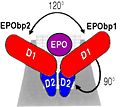
In the second interaction site, the Methionine 150 allows van der waals interactions with Arginine 10, the Valine 11 and the Arginine 14 of Erythropoietin. | In the second interaction site, the Methionine 150 allows van der waals interactions with Arginine 10, the Valine 11 and the Arginine 14 of Erythropoietin. | ||
| - | The hydrophobic interaction include 11 hydrogen bonds between αA and αC of Erythropoietin and his receptor. | + | The hydrophobic interaction include 11 hydrogen bonds between αA and αC of Erythropoietin and his receptor. |
| Line 89: | Line 89: | ||
== References == | == References == | ||
<references/> | <references/> | ||
| - | <references/>Por-Hsiung Lai, RicharEd verett, Fung-Fang Wang, Tsutomu Arakawa, and Eugene Goldwas, Structural Characterization of Human Erythropoietin, THE JOURNAL OF BIOLOGICAL CHEMISTRY (1986) vol.261 N°7 3116-3121 | ||
Revision as of 10:49, 24 December 2011
Contents |
HUMAN ERYTHROPOIETIN
|
The Erythropoietin hormone is a glycoprotein which is involved in Erythropoiesis, which is the red blood cells production [1]. It allows the differenciation of the erythrocyte precursors in the bone marrow. Thus, Epo has a fundamental biological role in the regulation of the production of red blood cells and, thus, at the level of the oxygenation of the blood.
Biological role
The gene of the erythropoietin was cloned at the man's, the mouse and the monkey. It is located at the man's on the chromosome 7 in the region q21. It contains 5 exons and 4 introns. The gene is very preserved according to the species. It exists 94 % of homology between the human gene and the monkey gene and 80 % of homology enters the murin gene and the human gene. L'Erythropoietin is a glycosylated hormone mainly secreted in the kidneys (90%) at the level of the cortex and of external medullary, and little in the liver. This hormone acts on the target cells by the intermediary of a specific receptor essentially on the erythroblastic progenitor, and a little on megacaryocyte progenitor. Through experiences of connection with the market hormone, it was demonstrated the existence of a single receptor (high affinity) or two types of receptor of high and low affinity. After the binding with its receptor, the Erythropoietin specifically leads the phosphorylation of the receptor on the tyrosine, from the first minutes which follow its connection.
The secretion of this hormone depend of the partial pressure of dioxygen in the kidney cells. The rate of Erythropoietin increases when there is a tissular hypoxie which origin can be anemic, secondary in an obstructive lung syndrome, in a left-right cardiac shunt or, much more rarely, in an abnormality of the affinity of the haemoglobin for oxygen. Thus needs of dioxygen of the tissues increase and thus the secretion of Erythropoietin is stimulated.
On the contrary, an increase of the pressure of dioxygen decrease the secretion of Erythropoietin.
To increase the number of red blood cells, this hormone stimulate the proliferation of stem cells precursor of erythrocyte in the bone marrow which will became red blood cells.
For regulate the expression of the Erythropoietin, there are activating regulating sequences in 3 ' of the gene as well as the sequences in 5 ' which are specific of tissue. On the other hand, recent works suggest that a heminique protein would play a regulating role on the transcription: if the tissular oxygenation is adequate, this protein fixes the oxygen and inhibits the transcription of Erythropoietin.
In the absence of oxygen, the deoxygenated shape of this heminique protein could lead the production of the Erythropoietin. The glycosylation of this hormone allows his solubilisation in the blood and his carry towards his target, the bone marrow.
Structure
| |||||||||||
αα
Application in medicine
See also
References
- ↑ Wolfgang Jelkmann and Eric Metzen, Erythropoietin in the control of red cell production, ANNALS Of ANATOMY
- ↑ Douglas D. Banks, The Effect of Glycosylation on the Folding Kinetics of Erythropoietin, J. Mol. Biol. (2011) 412, 536–550
- ↑ Por-Hsiung Lai, RicharEd verett, Fung-Fang Wang, Tsutomu Arakawa, and Eugene Goldwas, Structural Characterization of Human Erythropoietin, THE JOURNAL OF BIOLOGICAL CHEMISTRY (1986) vol.261 N°7 3116-3121
- ↑ Boissel JP, Lee WR, Presnell SR, Cohen FE, Bunn HF. Erythropoietin structure-function relationships. Mutant proteins that test a model of tertiary structure. J Biol Chem. 1993 Jul 25;268(21):15983-93. PubMed PMID:8340419
- ↑ Syed, Rashid S., Scott W. Reid, Cuiwei Li, Janet C. Cheetham, Kenneth H. Aoki, Beishan Liu, Hangjun Zhan, Timothy D. Osslund, Arthur J. Chirino, Jiandong Zhang, Janet Finer-Moore, Steven Elliott, Karen Sitney, Bradley A. Katz, David J. Matthews, John J. Wendoloski, Joan Egrie, and Robert M. Stroud. 1998. Efficiency of Signalling through Cytokine Receptors Depends Critically on Receptor Orientation. Nature 395: 511-516.