Hemochromatosis protein
From Proteopedia
(Difference between revisions)
| Line 9: | Line 9: | ||
== Description of HFE structure == | == Description of HFE structure == | ||
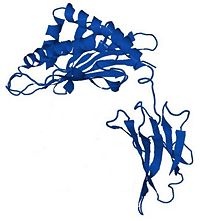
| - | <Structure load='1a6z' size='500' frame='true' align='right' caption=' | + | <Structure load='1a6z' size='500' frame='true' align='right' caption='1a6z, [[resolution]] 2.60 Å (<scene name='Sandbox_116_-_Human_Hemochromatosis_Protein_(1a6z)/Hfe_et_b2m/1'>default scene</scene>)' scene='Sandbox_116_-_Human_Hemochromatosis_Protein_(1a6z)/Hfe_et_b2m/1' /> |
HFE is a transmembrane glycoprotein composed by 343 amino acids. The gene coding for this protein is localized on the short arm of the 6th chromosome.<br><br> | HFE is a transmembrane glycoprotein composed by 343 amino acids. The gene coding for this protein is localized on the short arm of the 6th chromosome.<br><br> | ||
| - | HFE is a MHC-class-1-like protein. In fact, it's composed by three extracellular domain : α1, α2 and α3, a transmembrane domain and a short cytoplasmic domain. <ref name="gao">PMID:19254567</ref> The α1 and the α2 domains form a platform composed of eight antiparallel β strands topped by two antiparallel α helices. <ref name="lebron">PMID:9546397</ref> Moreover, as every MHC class 1 protein, it can | + | HFE is a MHC-class-1-like protein. In fact, it's composed by three extracellular domain : α1, α2 and α3, a transmembrane domain and a short cytoplasmic domain. <ref name="gao">PMID:19254567</ref> The α1 and the α2 domains form a platform composed of eight antiparallel β strands topped by two antiparallel α helices. <ref name="lebron">PMID:9546397</ref> Moreover, as every MHC class 1 protein, it can bind to a microglobulin : β2-microglobulin. This interaction is indispensable for HFE activity.<br><br> |
HFE has a 2 identified interactors. It can bind Transferrin Receptor 1 (TfR1) through its α1 and α2 domains. HFE can also bind Transferrin Receptor 2 (TfR2) through its α3 domain. Transferrin is an iron carrier. To understand better the mechanism in which HFE is implicated, we also have to know that on TfR1, the binding sites of HFE and Holo-Tf (that's to say transferrin without iron) are overlaping. <ref name="gao"/><br><br> | HFE has a 2 identified interactors. It can bind Transferrin Receptor 1 (TfR1) through its α1 and α2 domains. HFE can also bind Transferrin Receptor 2 (TfR2) through its α3 domain. Transferrin is an iron carrier. To understand better the mechanism in which HFE is implicated, we also have to know that on TfR1, the binding sites of HFE and Holo-Tf (that's to say transferrin without iron) are overlaping. <ref name="gao"/><br><br> | ||
HFE and TfR1 are forming a high affinity complex at the slighly basic pH found at the cell surface (pH = 7,5), but at acidic pH corresponding to intracellular vesicles, there is almost no binding between HFE and TfR. That may be because of the histidin residues located at the surface of the protein. Because of their pKa near 7, histidin are likely to be neutral at a basic pH and to carry a positive charge at an acid pH. <ref name="lebron"/> | HFE and TfR1 are forming a high affinity complex at the slighly basic pH found at the cell surface (pH = 7,5), but at acidic pH corresponding to intracellular vesicles, there is almost no binding between HFE and TfR. That may be because of the histidin residues located at the surface of the protein. Because of their pKa near 7, histidin are likely to be neutral at a basic pH and to carry a positive charge at an acid pH. <ref name="lebron"/> | ||
Revision as of 19:54, 26 December 2011
This sandbox is reserved by Lucie H, ESBS, for a Proteopedia project. Please don't modify anything !
Iron is indispensable for a lot of biological mechanisms in the organism, as a cofactor for many enzymes. However, an overload of this metal is toxic and can trigger serious diseases, such as hemochromatosis. So the iron absorption in the organism is an important mechanism that has to be precisely regulated. The human hemochromatosis protein (HFE) plays a major role in this mechanism.
Contents |
Description of HFE structure
| |||||||||||
Proteopedia Page Contributors and Editors (what is this?)
Lucie Hartmann, Michal Harel, Wayne Decatur, Jaime Prilusky, Nathalie Faggianelli