Sandbox 35
From Proteopedia
| Line 20: | Line 20: | ||
Papain. Lights. Camera. Action! | Papain. Lights. Camera. Action! | ||
| - | <StructureSection load='9pap' size='500' side='right' caption='Structure of HMG-CoA reductase (PDB entry [[9pap]])' scene=''> | ||
==Structure== | ==Structure== | ||
| - | Papain's single polypeptide chain consists of 212 amino acid residues which fold to form a groove containing the active site between its two domains. Its | + | <StructureSection load='9pap' size='500' side='right' caption='Structure of HMG-CoA reductase (PDB entry [[9pap]])' scene=''> |
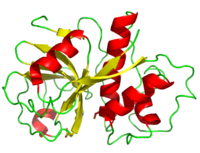
| - | <scene name='Sandbox_35/Secondary_structure_papain/2'>secondary structure</scene> consists of 17 <scene name='Sandbox_35/2nd_struc_papain_beta/2'>beta sheet</scene> strands and 7 <scene name='Sandbox_35/2nd_struc_papain_helix/2'>alpha helices</scene> giving it a composition 21% and 25% respectively. <ref name="9PAP PDB">[http://www.pdb.org/pdb/explore/explore.do?structureId=9PAP]9PAP PDB</ref> The hydrogen bonds within the alpha helices are shorter than the typical alpha helix because of C=O being directed further away from the helical axis. Moreover, the beta sheet hydrogen bonding constraints and structural angles show great variation; hydrogen bonds in the sheets' central tend to be shorter than on the fringes. Three disulfide bonds, like <scene name='Sandbox_35/Papain_cys_bond/1'>Cys 22-Cys 63</scene>, serve to hold papain's tertiary structure together. <ref name="Kamphuis">PMID: 6502713</ref> | + | ==Structural Elements== |
| + | Papain's single polypeptide chain consists of 212 amino acid residues which fold to form a groove containing the active site between its two domains. Its <scene name='Sandbox_35/Secondary_structure_papain/2'>secondary structure</scene> consists of 17 <scene name='Sandbox_35/2nd_struc_papain_beta/2'>beta sheet</scene> strands and 7 <scene name='Sandbox_35/2nd_struc_papain_helix/2'>alpha helices</scene> giving it a composition 21% and 25% respectively. <ref name="9PAP PDB">[http://www.pdb.org/pdb/explore/explore.do?structureId=9PAP]9PAP PDB</ref> The hydrogen bonds within the alpha helices are shorter than the typical alpha helix because of C=O being directed further away from the helical axis. Moreover, the beta sheet hydrogen bonding constraints and structural angles show great variation; hydrogen bonds in the sheets' central tend to be shorter than on the fringes. Three disulfide bonds, like <scene name='Sandbox_35/Papain_cys_bond/1'>Cys 22-Cys 63</scene>, serve to hold papain's tertiary structure together. <ref name="Kamphuis">PMID: 6502713</ref> | ||
| - | Located in the cleft between its domains, the active site consists of seven subsites (S1-S4 and S1’-S3’) each accommodating one amino acid residue of a substrate (P1-P4 and P1’-P3’). <ref>Schechter and Berger 1967</ref> The specificity of the active site is controlled by the S2 subsite which is a hydrophobic pocket that accommodates the P2 side chain of the substrate. Particularly at this subsite, papain shows specific substrate preferences for bulky hydrophobic or aromatic residues. On the other hand, outside of the S2 subsite preferences, the active site appears to exhibit a lack of clearly defined residue selectivity from within. <ref>Kimmel and Smith 1954</ref> | + | Located in the cleft between its domains, the active site consists of seven subsites (S1-S4 and S1’-S3’) each accommodating one amino acid residue of a substrate (P1-P4 and P1’-P3’). <ref>Schechter and Berger 1967</ref> The specificity of the active site is controlled by the S2 subsite which is a hydrophobic pocket that accommodates the P2 side chain of the substrate. Particularly at this subsite, papain shows specific substrate preferences for bulky hydrophobic or aromatic residues. On the other hand, outside of the S2 subsite preferences, the active site appears to exhibit a lack of clearly defined residue selectivity from within. <ref>Kimmel and Smith 1954</ref> |
| Line 45: | Line 45: | ||
| - | <scene name='Sandbox_35/Cathepsin_l_specific_inhibitor/3'>Cathepsin L specific inhibitor</scene> is part of a series known as CLIK inhibitors and was used on papain as an assessment of inhibition specificity for cathepsin enzymes. Structural differences between Papain-CLIK 148 complex and original papain is not very drastic. Minute changes result primarily from alterations in surface proteins except where a covalent bond is formed between the C2 on <scene name='Sandbox_35/Clik_cys/1'>CLIK 148 and Cys 25 residue</scene>. The primary <scene name='Sandbox_35/Cathepsin_interaction/3'>interactions</scene> between pseudo-substrate/inhibitor and papain were non-water hydrogen bonds and mostly hydrophobic interactions. CLIK 148's binding to the active site of papain is in a non-substrate mode with the main site showing pyrimidine ring interaction between <scene name='Sandbox_35/Clik_trp_177/1'>Trp 177 and CLIK 148</scene>. Hydrogen bonding is observed between the oxygens in <scene name='Sandbox_35/Clik_gly_gln/1'>CLIK 148 to Gln 19 and Gly 66 residues</scene>. Moreover, a water molecule has been observed to be near the His 159 residue enabling greater hydrogen bonding, once again highlighting solvents role in stability. <ref>PMID: 10600517</ref> | + | <scene name='Sandbox_35/Cathepsin_l_specific_inhibitor/3'>Cathepsin L specific inhibitor</scene> is part of a series known as CLIK inhibitors and was used on papain as an assessment of inhibition specificity for cathepsin enzymes. Structural differences between Papain-CLIK 148 complex and original papain is not very drastic. Minute changes result primarily from alterations in surface proteins except where a covalent bond is formed between the C2 on <scene name='Sandbox_35/Clik_cys/1'>CLIK 148 and Cys 25 residue</scene>. The primary <scene name='Sandbox_35/Cathepsin_interaction/3'>interactions</scene> between pseudo-substrate/inhibitor and papain were non-water hydrogen bonds and mostly hydrophobic interactions. CLIK 148's binding to the active site of papain is in a non-substrate mode with the main site showing pyrimidine ring interaction between <scene name='Sandbox_35/Clik_trp_177/1'>Trp 177 and CLIK 148</scene>. Hydrogen bonding is observed between the oxygens in <scene name='Sandbox_35/Clik_gly_gln/1'>CLIK 148 to Gln 19 and Gly 66 residues</scene>. Moreover, a water molecule has been observed to be near the His 159 residue enabling greater hydrogen bonding, once again highlighting solvents role in stability. <ref>PMID: 10600517</ref> More favorable energetic has also been revealed through modeling when hydrophobic and aromatic parts of the ligand occupying the S2, S3, and S1' subsites with at least three hydrogen bonding contacts between the protein conserved binding site residues and the ligand. <ref>PMID: 9472614</ref> |
</StructureSection> | </StructureSection> | ||
| Line 61: | Line 61: | ||
[[Image:Papain Simple Cleavage.jpg|200px|right|thumb|Simple Overview of Papain Cleavage. <ref>[http://www.worthington-biochem.com/pap/default.html] Worthington Biochemical Corporation </ref>]] | [[Image:Papain Simple Cleavage.jpg|200px|right|thumb|Simple Overview of Papain Cleavage. <ref>[http://www.worthington-biochem.com/pap/default.html] Worthington Biochemical Corporation </ref>]] | ||
| - | Except for valine, papain prefers to cleave at hydrophobic residues alanine, leucine, isoleucine, phenylalanine, tryptophan, or tyrosine <ref>[http://www.sigmaaldrich.com/life-science/biochemicals/biochemical-products.html?TablePage=16410606] | + | Except for valine, papain prefers to cleave at hydrophobic residues alanine, leucine, isoleucine, phenylalanine, tryptophan, or tyrosine <ref>[http://www.sigmaaldrich.com/life-science/biochemicals/biochemical-products.html?TablePage=16410606] Sigma Aldrich Papain</ref>. In light of the describe catalytic mechanism it makes sense that substances such as cysteine, sulfide/sulfite, heavy metal chelating agents like EDTA, and N-bromosuccinimide act as activators of the enzyme while PMSF, Hg2+ and other heavy metals, cystatin, leupeptin, sulfhydryl binding agents, carbonyl reagents, and alkylating agents serve as inhibitors. |
Revision as of 20:04, 8 March 2012
| Please do NOT make changes to this Sandbox. Sandboxes 30-60 are reserved for use by Biochemistry 410 & 412 at Messiah College taught by Dr. Hannah Tims during Fall 2012 and Spring 2013. |
Contents |
Papain
Introduction
Did you know?
. Meat tenderizer. Old time home remedy for insect, jellyfish, and stingray stings[1]. Who would have thought that a sulfhydryl protease from the latex of the papaya fruit, Carica papaya and Vasconcellea cundinamarcensis would have such a practical application beyond proteopedia?
This protease belongs to an extended family of aminopeptidases, dipeptidyl peptidases, endopeptidases, and other enzymes having both exo- and endo-peptidase activity. The inactivated zymogen with N-terminal propeptide regions - providing stability in alkaline environments and enabling proper folding - is activated through removal of the propeptide regions. [2] The protein is primarily secreted with its pro-region enabling transport from zymogen to lysosome through membrane association and mediation. [3]
Historicity
Papain made its first appearance in the Calcutta Medical Journal entitled “The Solvent Action of Papaya Juice on Nitrogenous Articles of Food” when G.C Roy was investigating the enzyme in 1873. In the late 19th century, Wurtz and Bouchut dubbed the partially purified enzyme "papain." [4] At the time, it was viewed as proteolytically active constituent in the latex of tropical papaya fruit. [5] As separation and purification techniques improved, pure papain was able to be isolated. In becoming the second enzyme to attain an X-ray crystallized structure and the first cysteine protease to behold an identifiable structure, papain fueled greater advances in enzymatic studies. [6]
Papain. Lights. Camera. Action!
Structure
| |||||||||||
Catalytic Mechanism
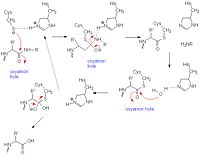
Papain's catalytic mechanism is like serine proteases. Its catalytic triad of residues Cys 25- His159- Asn-175 appear to work with a fourth residue, Gln-19, suspected to be involved in oxyanion hole formation. When a peptide binds to the active site, His-159 deprotonates Cys-25 which in turn attacks the substrate carbonyl carbon. The oxyanion hole then stabilizes the resulting covalent, tetrahedral intermediate. Subsequently, nitrogen in the peptide bond is protonated by His-159 (acting as an acid). This action frees the C-terminal portion of the peptide so that it is released. The entrance of water into the active site then attacks the carbonyl carbon while it is deprotonated by His-159, resulting in another tetrahedral covalent intermediate once again stabilized through the oxyanion hole. At the end, carbonyl reformation and the Cys-25 sulfur action as the leaving group releases the N-terminal portion of the peptide. The enzyme is regenerated for the cycle to begin again. [18]
Other Interaction with Inhibitors and Effectors
Except for valine, papain prefers to cleave at hydrophobic residues alanine, leucine, isoleucine, phenylalanine, tryptophan, or tyrosine [20]. In light of the describe catalytic mechanism it makes sense that substances such as cysteine, sulfide/sulfite, heavy metal chelating agents like EDTA, and N-bromosuccinimide act as activators of the enzyme while PMSF, Hg2+ and other heavy metals, cystatin, leupeptin, sulfhydryl binding agents, carbonyl reagents, and alkylating agents serve as inhibitors.
Fun Trivia
Remember the 2002 SARS (Severe Acute Respiratory Syndrome) epidemic that placed global health, particularly in Southeast Asia, in a precarious state? On-going research is happening to further understand the mechanisms of this coronavirus, so that future steps can be taken for prevention. Its been found that the replication of RNA for this virus is mediated by two viral proteases that have many papain-like characteristics! [21]
References
- ↑ [1] Ameridan International
- ↑ Rawlings ND, Barrett AJ. Families of cysteine peptidases. Methods Enzymol. 1994;244:461-86. PMID:7845226
- ↑ Yamamoto Y, Kurata M, Watabe S, Murakami R, Takahashi SY. Novel cysteine proteinase inhibitors homologous to the proregions of cysteine proteinases. Curr Protein Pept Sci. 2002 Apr;3(2):231-8. PMID:12188906
- ↑ Menard and Storer 1998
- ↑ Wurtz and Bouchut 1879
- ↑ Drenth J, Jansonius JN, Koekoek R, Swen HM, Wolthers BG. Structure of papain. Nature. 1968 Jun 8;218(5145):929-32. PMID:5681232
- ↑ [2]9PAP PDB
- ↑ 8.0 8.1 8.2 8.3 Kamphuis IG, Kalk KH, Swarte MB, Drenth J. Structure of papain refined at 1.65 A resolution. J Mol Biol. 1984 Oct 25;179(2):233-56. PMID:6502713
- ↑ Schechter and Berger 1967
- ↑ Kimmel and Smith 1954
- ↑ 11.0 11.1 Wang J, Xiang YF, Lim C. The double catalytic triad, Cys25-His159-Asp158 and Cys25-His159-Asn175, in papain catalysis: role of Asp158 and Asn175. Protein Eng. 1994 Jan;7(1):75-82. PMID:8140097
- ↑ Ménard R, Khouri HE, Plouffe C, Dupras R, Ripoll D, Vernet T, Tessier DC, Lalberté F, Thomas DY, Storer AC. A protein engineering study of the role of aspartate 158 in the catalytic mechanism of papain. Biochemistry. 1990 Jul 17;29(28):6706-13. PMID:2397208 doi:10.1021/bi00480a021
- ↑ [3] The Journal of Biological Chemistry
- ↑ 14.0 14.1 [4] Jane S. Richardson
- ↑ [5] WebMD
- ↑ Tsuge H, Nishimura T, Tada Y, Asao T, Turk D, Turk V, Katunuma N. Inhibition mechanism of cathepsin L-specific inhibitors based on the crystal structure of papain-CLIK148 complex. Biochem Biophys Res Commun. 1999 Dec 20;266(2):411-6. PMID:10600517 doi:10.1006/bbrc.1999.1830
- ↑ Skern T, Fita I, Guarne A. A structural model of picornavirus leader proteinases based on papain and bleomycin hydrolase. J Gen Virol. 1998 Feb;79 ( Pt 2):301-7. PMID:9472614
- ↑ 18.0 18.1 [6] University of Maine
- ↑ [7] Worthington Biochemical Corporation
- ↑ [8] Sigma Aldrich Papain
- ↑ Barretto N, Jukneliene D, Ratia K, Chen Z, Mesecar AD, Baker SC. The papain-like protease of severe acute respiratory syndrome coronavirus has deubiquitinating activity. J Virol. 2005 Dec;79(24):15189-98. PMID:16306590 doi:10.1128/JVI.79.24.15189-15198.2005