Papain
From Proteopedia
| Line 44: | Line 44: | ||
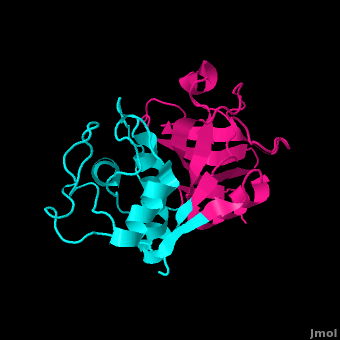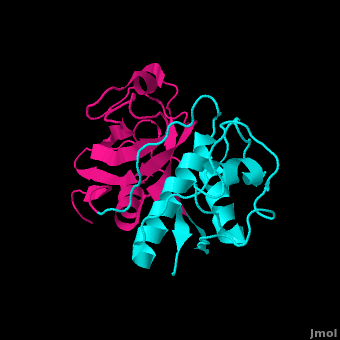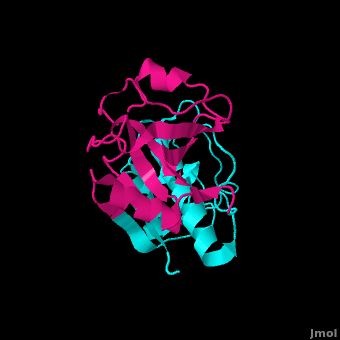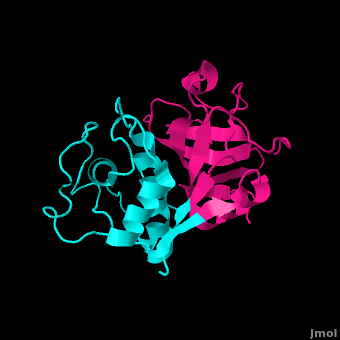
==='''Cathepsin L (PDB ID #: 1CVZ)'''=== | ==='''Cathepsin L (PDB ID #: 1CVZ)'''=== | ||
| - | Cathepsin L is another inhibitor of the papain enzyme. Cathepsin L interacts with the <scene name='Sandbox_31/Cathepl/1'>papain residues</scene> Gln19, Cys25, Gly66, Asp158, and Trp177 by hydrogen bonding them (Cathepsin L is lime green in the model, and the papain residues active in hydrogen bonding with Cathepsin L are highlighted with yellow halos). In addition to hydrogen bonding, hydrophobic interactions exist to exclude water, allowing the papain enzyme and Cathepsin L to associate even closer. Finally, <scene name='Sandbox_31/Stacking/1'>Ring Stacking</scene> between Trp177 and the Capthespin L molecule hold them tightly together.<ref> | + | Cathepsin L is another inhibitor of the papain enzyme. Cathepsin L interacts with the <scene name='Sandbox_31/Cathepl/1'>papain residues</scene> Gln19, Cys25, Gly66, Asp158, and Trp177 by hydrogen bonding them (Cathepsin L is lime green in the model, and the papain residues active in hydrogen bonding with Cathepsin L are highlighted with yellow halos). In addition to hydrogen bonding, hydrophobic interactions exist to exclude water, allowing the papain enzyme and Cathepsin L to associate even closer. Finally, <scene name='Sandbox_31/Stacking/1'>Ring Stacking</scene> between Trp177 and the Capthespin L molecule hold them tightly together.<ref>PMID:18598021</ref> Cathepsin L plays a roll in many different diseases including malaria, leishmaniasis, Chagas' disease, African trypanosomiasis, toxoplasmosis, and amoebiasis.<ref>http://www.ncbi.nlm.nih.gov/pmc/articles/PMC2923042/?tool=pubmed</ref>Some studies show that there is a relation between cathepsins and certain cancers, alzheimer's, and arthritis.<ref>http://www.biomedcentral.com/1472-6807/10/30</ref> |
==='''Stefin B'''=== | ==='''Stefin B'''=== | ||
Revision as of 03:46, 5 April 2012

Papain. Meat tenderizer. Old time home remedy for insect, jellyfish, and stingray stings[2]. Who would have thought that a sulfhydryl protease from the latex of the papaya fruit, Carica papaya and Vasconcellea cundinamarcensis would have such a practical application beyond proteopedia?
Papain is a 23.4 kDa, 212 residue cysteine protease, also known as papaya proteinase I, from the peptidase C1 family (E.C. 3.4.22.2).[3][4] It is the natural product of the Papaya(Carica papaya)[5], and may be extracted from the plant's latex, leaves and roots. [6]. Papain displays a broad range of functions, acting as an endopeptidase, exopeptidase, amidase, and esterase,[6] with its optimal activity values for pH lying between 6.0 and 7.0, and its optimal temperature for activity is 65 °C. Its pI values are 8.75 and 9.55, and it is best visualized at a wavelength of 278 nm. [5]
Papain's enzymatic use was first discovered in 1873 by G.C. Roy who published his results in the Calcutta Medical Journal in the article, "The Solvent Action of Papaya Juice on Nitrogenous Articles of Food." In 1879, papain was named officially by Wurtz and Bouchut, who managed to partially purify the product from the sap of papaya. It wasn't until the mid-twentieth century that the complete purification and isolation of papain was achieved. In 1968, Drenth et al. determined the structure of papain by x-ray crystallography, making it the second enzyme whose structure was successfully determined by x-ray crystallography. Additionally, papain was the first cysteine protease to have its structure identified.[6] In 1984, Kamphuis et al. determined the geometry of the active site, and the three-dimensional structure was visualized to a 1.65 Angstrom solution.[7] Today, studies continue on the stability of papain, involving changes in environmental conditions as well as testing of inhibitors such as phenylmethanesulfonylfluoride (PMSF), TLCK, TPCK, aplh2-macroglobulin, heavy metals, AEBSF, antipain, cystatin, E-64, leupeptin, sulfhydryl binding agents, carbonyl reagents, and alkylating agents.[6]
Contents |
Structure
| |||||||||||
Inhibitors
| |||||||||||
Common Uses
Medicinal
Papain has been used for a plethora of medicinal purposes including treating inflammation, shingles, diarrhea, psoriasis, parasites, and many others.[22] One major use is the treatment of cutaneous ulcers including diabetic ulcers and pressure ulcers.[23] Pressures ulcers plague many bed bound individuals and are a major source of pain and discomfort. Two papain based topical drugs are Accuzyme and Panafil, which can be used to treat wounds like cutaneous ulcers.[24]
A recent New York Times article featured papain and other digestive enzymes.[25] With the number of individuals suffering from irritable bowel syndrome and other gastrointestinal issues, many people are turning toward natural digestive aid supplements like papain. The author even talks about the use of papain along with a pineapple enzyme, bromelain, in cosmetic facial masks. Dr. Adam R. Kolker (a plastic surgeon) is quoted in the article saying that "For skin that is sensitive, enzymes are wonderful." He bases these claims off the idea that proteases like papain help to break peptide bonds holding dead skin cells to the live skin cells.[26]
Commercial and Biomedical
Papain digests most proteins, often more extensively than pancreatic proteases. It has a very broad specificity and is known to cleave peptide bonds of basic amino acids and leucine and glycine residues, but prefers amino acids with large hydrophobic side chains. This non-specific nature of papain's hydrolase activity has led to its use in many and varied commercial products. It is often used as a meat tenderizer because it can hydrolyze the peptide bonds of collagen, elastin, and actomyosin. It is also used in contact lens solution to remove protein deposits on the lenses and marketed as a digestive supplement. [27] Finally, papain has several common uses in general biomedical research, including a gentle cell isolation agent, production of glycopeptides from purified proteoglycans, and solubilization of integral membrane proteins. It is also notable for its ability to specifically cleave IgG and IgM antibodies above and below the disulfide bonds that join the heavy chains and that is found between the light chain and heavy chain. This generates two monovalent Fab segments, that each have a single antibody binding sites, and an intact Fc fragment.[6]
Fun Trivia
Remember the 2002 SARS (Severe Acute Respiratory Syndrome) epidemic that placed global health, particularly in Southeast Asia, in a precarious state? On-going research is happening to further understand the mechanisms of this coronavirus, so that future steps can be taken for prevention. Its been found that the replication of RNA for this virus is mediated by two viral proteases that have many papain-like characteristics. [28]
Despite a low percentage of sequence identities, inhibition and sequence analyses have increasingly been drawing parallels between L proteinases, that involve the foot-and-mouth disease virus and equine rhinovirus 1, and papain. With a similar overall fold to papain and identifiable regions that resemble the five alpha-helices and seven beta-sheets of papain, L proteinases of foot-and-mouth disease virus and of equine rhinovirus 1 reveal a mode of operation that is very papain like. [29]
References
- ↑ [1] Papaya's Nutrition Facts
- ↑ [2] Ameridan International
- ↑ [3] Uniprot
- ↑ 4.0 4.1 [4] 9PAP PDB
- ↑ 5.0 5.1 5.2 [5] Sigma Aldrich
- ↑ 6.0 6.1 6.2 6.3 6.4 [6] Worthington
- ↑ 7.0 7.1 Kamphuis IG, Kalk KH, Swarte MB, Drenth J. Structure of papain refined at 1.65 A resolution. J Mol Biol. 1984 Oct 25;179(2):233-56. PMID:6502713
- ↑ [7] Jane S. Richardson
- ↑ [8] The Structure of Papain
- ↑ [9] RCSB PDB
- ↑ Berger A, Schechter I. Mapping the active site of papain with the aid of peptide substrates and inhibitors. Philos Trans R Soc Lond B Biol Sci. 1970 Feb 12;257(813):249-64. PMID:4399049
- ↑ [10]Shokhen M, N Khazanov, and A Albeck. 2009. Challenging a paradigm: theoretical calculations of the protonation state of the Cys25-His159 catalytic diad in free papain. Proteins. 77(4):916-26.
- ↑ [11]Noble MA, Gul S, Verma CS, Brocklehurst K. 2000. Ionization characteristics and chemical influences of aspartic acid residue 158 of papain and caricain determined by structure-related kinetic and computational techniques: multiple electrostatic modulators of active-centre chemistry. Biochem J. 2000 351: 723-33.
- ↑ 14.0 14.1 [12] University of Maine
- ↑ [13] Harrison, M.J., N.A. Burton, and I.H. Hillier. 1997. Catalytic Mechanism of the Enzyme Papain: Predictions with a Hybrid Quantum Mechanical/Molecular Mechanical Potential. J. Am. Chem. Soc. 119: 12285-12291
- ↑ [14] Schröder, E., C. Phillips, E. Garman, K. Harlos, C. Crawford. 1997. X-ray crystallographic structure of a papain-leupeptin complex. FEBS Letters 315: 38-42
- ↑ LaLonde JM, Zhao B, Smith WW, Janson CA, DesJarlais RL, Tomaszek TA, Carr TJ, Thompson SK, Oh HJ, Yamashita DS, Veber DF, Abdel-Meguid SS. Use of papain as a model for the structure-based design of cathepsin K inhibitors: crystal structures of two papain-inhibitor complexes demonstrate binding to S'-subsites. J Med Chem. 1998 Nov 5;41(23):4567-76. PMID:9804696 doi:10.1021/jm980249f
- ↑ Beavers MP, Myers MC, Shah PP, Purvis JE, Diamond SL, Cooperman BS, Huryn DM, Smith AB 3rd. Molecular docking of cathepsin L inhibitors in the binding site of papain. J Chem Inf Model. 2008 Jul;48(7):1464-72. Epub 2008 Jul 4. PMID:18598021 doi:10.1021/ci800085c
- ↑ http://www.ncbi.nlm.nih.gov/pmc/articles/PMC2923042/?tool=pubmed
- ↑ http://www.biomedcentral.com/1472-6807/10/30
- ↑ http://www.ncbi.nlm.nih.gov/pmc/articles/PMC551902/pdf/emboj00233-0254.pdf
- ↑ http://www.webmd.com/vitamins-supplements/ingredientmono-69-PAPAIN.aspx?activeIngredientId=69&activeIngredientName=PAPAIN
- ↑ http://www.pbm.va.gov/Clinical%20Guidance/Drug%20Monographs/Papain%20Urea.pdf
- ↑ http://www.pbm.va.gov/Clinical%20Guidance/Drug%20Monographs/Papain%20Urea.pdf
- ↑ http://www.nytimes.com/2012/02/23/fashion/enzymes-once-sidelined-try-to-grab-the-spotlight.html
- ↑ http://www.nytimes.com/2012/02/23/fashion/enzymes-once-sidelined-try-to-grab-the-spotlight.html
- ↑ http://www.webmd.com/vitamins-supplements/ingredientmono-69-PAPAIN.aspx?activeIngredientId=69&activeIngredientName=PAPAIN
- ↑ Barretto N, Jukneliene D, Ratia K, Chen Z, Mesecar AD, Baker SC. The papain-like protease of severe acute respiratory syndrome coronavirus has deubiquitinating activity. J Virol. 2005 Dec;79(24):15189-98. PMID:16306590 doi:10.1128/JVI.79.24.15189-15198.2005
- ↑ Skern T, Fita I, Guarne A. A structural model of picornavirus leader proteinases based on papain and bleomycin hydrolase. J Gen Virol. 1998 Feb;79 ( Pt 2):301-7. PMID:9472614
Reference
- Wang J, Xiang YF, Lim C. The double catalytic triad, Cys25-His159-Asp158 and Cys25-His159-Asn175, in papain catalysis: role of Asp158 and Asn175. Protein Eng. 1994 Jan;7(1):75-82. PMID:8140097
- Kamphuis IG, Kalk KH, Swarte MB, Drenth J. Structure of papain refined at 1.65 A resolution. J Mol Biol. 1984 Oct 25;179(2):233-56. PMID:6502713
3D Structures of Papain
3LFY, 1KHP, 1KHQ, 1CVZ, 1BQI, 1BP4, 1PPN, 1PPP, 1PIP, 1POP, 1PE6, 9PAP, 1PPD, 1PAD, 2PAD, 4PAD, 5PAD, 6PAD- Carica papaya
3IMA - Colocasia esculenta
1STF - Homo sapiens
2CIO - Trypanosoma brucei
3E1Z - Trypanosoma cruzi
Page seeded by OCA on Tue Feb 17 04:20:31 2009
Proteopedia Page Contributors and Editors (what is this?)
Kirsten Eldredge, Jacinth Koh, Sara Kongkatong, Kyle Burch, Michal Harel, Joel L. Sussman, Elizabeth Miller, Samuel Bray, David Canner, Jaime Prilusky