Lipase
From Proteopedia
| Line 3: | Line 3: | ||
== '''Introduction''' == | == '''Introduction''' == | ||
| - | Lipase is a hydrolase that catalyzes the breakdown of lipids by hydrolyzing the esters of fatty acids. | + | Lipase is a hydrolase that catalyzes the breakdown of lipids by hydrolyzing the esters of fatty acids. This makes lipase important in digestion and promoting absorption of fats in the intestines. Lipase is primarily found in and secreted by the pancreas but is also found in the saliva and the stomach. Pancreatic lipase (PDB ID: 1HPL) which is pictured to the right is a carboxylic ester hydrolase. It is also commonly called pancreatic triacylglycerol lipase and its enzyme class number is E.C. 3.1.1.3 <ref name="1HPL PDB SUM">[http://www.ebi.ac.uk/thornton-srv/databases/cgi-bin/pdbsum/GetPage.pl?pdbcode=1hpl&template=main.html] 1HPL PDB SUM </ref>. The reaction catalyzed by this enzyme is shown below. |
[[Image:Picture 1.png]] | [[Image:Picture 1.png]] | ||
| - | Further breakdown ultimately results in 2-monoacylglycerols and free fatty acids <ref name= "A cross-linked complex between horse pancreatic lipase and colipase">[http://www.sciencedirect.com/science/article/pii/0014579389815923] A cross-linked complex between horse pancreatic lipase and colipase</ref>. | + | Further breakdown ultimately results in 2-monoacylglycerols and free fatty acids <ref name= "A cross-linked complex between horse pancreatic lipase and colipase">[http://www.sciencedirect.com/science/article/pii/0014579389815923] A cross-linked complex between horse pancreatic lipase and colipase</ref>. An in depth discussion of the mechanism can be found in the Lipase Catalytic Mechanism section. The determination of the structure and function of lipase was a gradual process. Lipase activity was first demonstrated in the pancreas by Claude Bernard in 1846. It wasn't until 1955 that Mattson and Beck demonstrated a high-specificity of pancreatic lipase for triglyceride primary esters <ref name= "History of Lipids">[http://www.cyberlipid.org/history/history1.htm] History of Lipids</ref>. In recent years, determination of the crystal structure of pancreatic lipase has become the focus and many scientists have worked to further this. See also [[Molecular Playground/Pancreatic Lipase]]. |
== '''Structure''' == | == '''Structure''' == | ||
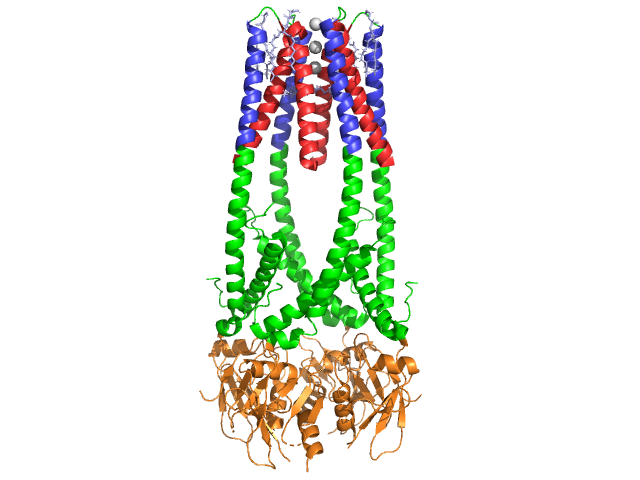
| - | + | Pancreatic lipase is a 50 kDa protein, consisting of two identical, 449 residue chains <ref name= "1HPL PDB">[http://www.pdb.org/pdb/explore/explore.do?structureId=1HPL] 1HPL PDB</ref>. The <scene name='Lipase/Secondary_structures/1'>secondary structure</scene>s of lipase (in one subunit) include 102 residues which create 13 alpha helices, shown in red, and 139 residues involved in beta sheets totaling 28 strands, shown in gold. The alpha helices account for 22% of the protein, while the beta sheets comprise 30%. Each chain contains two well defined <scene name='Lipase/N_and_c_terminus/1'>domains</scene>. The N terminal domain, shown in blue, is characterized by an alpha/beta hydrolase fold. While the C terminal domain, shown in green, contains a beta sheet sandwich which interacts with colipase <ref>http://www.pdb.org/pdb/explore/explore.do?structureId=1HPL</ref>. Each monomer and dimer structure of lipase is held together by disulfide bonds, hydrogen bonds, and electrostatic interactions (salt bridges). Lipase has 12 total <scene name='Lipase/Disulfide_bonds/2'>disulfide bonds</scene> between cysteine residues. <scene name='Lipase/Salt_bridges/1'>Salt bridges</scene> are formed between the positively charge nitrogens (blue) in Arg and Lys, and negative oxygens (red) in Asp and Glu residues. <scene name='Lipase/Hydrogen_bonds/2'>Hydrogen bonds</scene> (in yellow) also stabilize the enzyme between main chain and side chain atoms. Lipase has a distinct distribution of <scene name='Lipase/Hphobic_residues/3'>hydrophobic and hydrophilic</scene> residues (purple spacefill represents polar residues). Hydrophobic collapse contributes to much of the secondary and tertiary structures, as the <scene name='Lipase/Surface/1'>hydrophobic core residues</scene> (shown in white) make up the interior of the protein while polar residues (transparent blue) are on the surface <ref>http://www.pdb.org/pdb/explore/remediatedSequence.do?structureId=1HPL</ref>. In addition, lipase has two <scene name='Lipase/Lipase_ligand/1'>calcium ligands</scene>, one buried in each monomer subunit. The image shows the green calcium ion in subunit A, coordinated by Glu187, Arg190, Asp192, and Asp195. The Ca(+2) charge is stabilized by negatively charged glutamate and aspartate residues, and the oxygen atoms from two water molecules (pink). The calcium ion is essential to protein folding and enzyme activity <ref>http://www.springerlink.com/content/g5h1613440115701/fulltext.pdf</ref>. | |
| - | The <scene name='Lipase/Secondary_structures/1'>secondary structure</scene>s of lipase (in one subunit) include 102 residues which create 13 alpha helices, shown in red, and 139 residues involved in beta sheets totaling 28 strands, shown in gold. The alpha helices account for 22% of the protein, while the beta sheets comprise 30%. Each chain contains two well defined <scene name='Lipase/N_and_c_terminus/1'>domains</scene>. The N terminal domain, shown in blue, is characterized by an alpha/beta hydrolase fold. While the C terminal domain, shown in green, contains a beta sheet sandwich which interacts with colipase <ref>http://www.pdb.org/pdb/explore/explore.do?structureId=1HPL</ref>. Each monomer and dimer structure of lipase is held together by disulfide bonds, hydrogen bonds, and electrostatic interactions (salt bridges). Lipase has 12 total <scene name='Lipase/Disulfide_bonds/2'>disulfide bonds</scene> between cysteine residues. <scene name='Lipase/Salt_bridges/1'>Salt bridges</scene> are formed between the positively charge nitrogens (blue) in Arg and Lys, and negative oxygens (red) in Asp and Glu residues. <scene name='Lipase/Hydrogen_bonds/2'>Hydrogen bonds</scene> (in yellow) also stabilize the enzyme between main chain and side chain atoms. Lipase has a distinct distribution of <scene name='Lipase/Hphobic_residues/3'>hydrophobic and hydrophilic</scene> residues (purple spacefill represents polar residues). Hydrophobic collapse contributes to much of the secondary and tertiary structures, as the <scene name='Lipase/Surface/1'>hydrophobic core residues</scene> (shown in white) make up the interior of the protein while polar residues (transparent blue) are on the surface <ref>http://www.pdb.org/pdb/explore/remediatedSequence.do?structureId=1HPL</ref>. In addition, lipase has two <scene name='Lipase/Lipase_ligand/1'>calcium ligands</scene>, one buried in each monomer subunit. The image shows the green calcium ion in subunit A, coordinated by Glu187, Arg190, Asp192, and Asp195. The Ca(+2) charge is stabilized by negatively charged glutamate and aspartate residues, and the oxygen atoms from two water molecules (pink). The calcium ion is essential to protein folding and enzyme activity <ref>http://www.springerlink.com/content/g5h1613440115701/fulltext.pdf</ref>. | + | |
In addition, lipase has a unique <scene name='Lipase/Lid/2'>lid</scene> (green) that blocks solvent from entering the active site (red). The lid is a 25-residue helical structure protects the oxyanion hole. The lid (yellow) is especially important to substrate binding, as it undergoes a dramatic shift altering the secondary structure of lipase binding site from a <scene name='Lipase/Closed_lid/1'>closed lid structure</scene> (active site in red) to an <scene name='Lipase/Open_ring/1'>open ring structure</scene> (active site in blue, triacylglyceride in spacefill) <ref>Fundamentals of Biochemistry...</ref>. The lid opening is accompanied by a change in secondary structure from a mostly beta-extended confirmation to a structure where more than half the active site is formed from alpha helices <ref>Thomas, A. etc. "Role of the Lid Hydrophobicity Pattern in Pancreatic Lipase Activity", The Journal of Biological Chemistry, 2005 September 22; 270 (48): 40074-40083. </ref>. | In addition, lipase has a unique <scene name='Lipase/Lid/2'>lid</scene> (green) that blocks solvent from entering the active site (red). The lid is a 25-residue helical structure protects the oxyanion hole. The lid (yellow) is especially important to substrate binding, as it undergoes a dramatic shift altering the secondary structure of lipase binding site from a <scene name='Lipase/Closed_lid/1'>closed lid structure</scene> (active site in red) to an <scene name='Lipase/Open_ring/1'>open ring structure</scene> (active site in blue, triacylglyceride in spacefill) <ref>Fundamentals of Biochemistry...</ref>. The lid opening is accompanied by a change in secondary structure from a mostly beta-extended confirmation to a structure where more than half the active site is formed from alpha helices <ref>Thomas, A. etc. "Role of the Lid Hydrophobicity Pattern in Pancreatic Lipase Activity", The Journal of Biological Chemistry, 2005 September 22; 270 (48): 40074-40083. </ref>. | ||
Revision as of 14:35, 12 April 2012
| |||||||||||
3D Structures of Lipase
Update November 2011
Eukaryote natives:
1hpl – hLip – horse
1hlg – hLip – human - gastric
3jw8, 3hju – mono-glyceride hLip
1jmy – hBSSL
1akn – cBSSL – cattle
2bce - cBSSL (mutant)
1f6w - cBSSL – catalytic domain
3o0d – Lip – Yarrowia lipolytica
1gpl – Lip – Guinea pig
Prokaryote natives:
3guu, 1lbs, 1lbt, 1tca, 1tcb, 1tcc – CaLipA – Candida antarctica
2veo – CaLipA – closed state
3icv – CaLipB (mutant)
1gz7, 1lpm, 1lps– CrLip 2 – Candida rugosa - closed state
1crl, 1trh – CrLip – open state
1llf – Lip – Candida cylindracea
3g7n – Lip - Penicillium expansum
1tia - Lip – Penicillium camemberti
2qua, 2qub – LipA – Serratia marcescens
2hih – Lip – Staphylococcus hyicus
2fx5 – Lip – Pseudomonas mendocina
1yzf – Lip – Enterococcus faecalis
1dt3, 1dt5, 1dte, 1du4, 1ein, 1tib – TlLip - Thermomyces lanuginose
1jfr – Lip – Streptomyces exfoliates
1oil – BcLip - Burkholderia cepacia
2lip – BcLip – open state
1cvl – Lip – Chromobacterium viscosum
1lgy – Lip II – Rhizopus niveus
1tic - Lip – Rhizopus oryzae
1thg – Lip – Geotrichum candidum
3tgl, 4tgl, 1tgl – RmLip– Rhyzomucor miehei
2zvd – PsLip - Pseudomonas sp. – open state
2z8x - PsLip – extracellular
2zj6, 2zj7 – PsLip (mutant)
2z8z – PsLip(mutant) – closed state
3lip, 3a6z - Lip - Pseudomonas cepacia – open state
1qge, 1tah – Lip – Pseudomonas glumae
2w22 – Lip – Geobacillus thermocatenulatus
1ji3, 1ku0 – Lip – Bacillus stearothermophilus
1ah7 - Lip – Bacillus cereus
2qxt, 2qxu, 1isp, 1i6w - BsLip – Bacillus subtilis
3d2a, 3d2b, 3d2c, 1t2n, 1t4m, 3qmm - BsLip (mutant)
2ory – Lip – Photobacterium lypoliticum
2z5g, 2dsn – Lip T1 – Geobacillus zalihae
3p94 – Lip – Parabacteroides distasonis
3ngm – Lip – Gibberella zeae
Lipase/colipase complexes. The colipase is a co-enzyme whose binding to lipase optimizes the enzymatic activity
1n8s – hLip+colipase II
1eth, 1lpa - Lip+colipase II - pig
Hormone-sensitive-lipases (LIPE) hydrolyze the first fatty acid of the triacylglycerol substrate
3k6k – EstE7(LIPE) – metagenome library
3fak, 3dnm – EstE5(LIPE) – metagenome library
1evq – AaEst2(LIPE) – Alicyclobacillus acidocaldarius
1u4n – AaEst2(LIPE) (mutant)
Putative lipases; Proteins with unknown function but structural similarity to lipase obtained in structural genomics projects.
2rau - Lip – Sulfolobus solfataricus
3bxp, 3d3n - Lip – Lactobacillus plantarum
3e0x - Lip – Clostridium acetobutylicum
1z8h – Lip – Nostoc sp. PCC 712
1vj3 - Lip – Nostoc sp.
3bzw – Lip - Bacteroides thetaiotaomicron
2pbl – Lip - Silicibacter
Lipase + inhibitors
3jwe, 3pe6 - mono-glyceride hLip + SAR629 – covalent inhibitor
3l1h – EstE5(LIPE)+FeCl3 – noninvasive inhibitor
3l1i, 3l1j - EstE5(LIPE)+CuSO4 – noninvasive inhibitor
3lij - EstE5(LIPE)+ZnSO4– noninvasive inhibitor
3h18, 3h17 - EstE5 (LIPE)+PMSF
3h19, 3h1b, 3h1a – EstE5 (LIPE)+methyl alcohol
3h1a – EstE5 SLIPE)+ethyl alcohol
3h19 – EstE5 SLIPE)+isopropyl alcohol
3g9t, 3g9u - EstE5 (HSLIPE)+p-nitrophenyl butyrate
3g9z - EstE5 (LIPE) +p-nitrophenyl caprylate
2nw6 – BcLip+ S inhibitor
4lip, 5lip, 1r4z, 1r50 – BcLip+ Rc-(Rp,Sp)-1,2-dioctylcarbamoyl-glycero-3-O-phosphonate
1r4z – BsLip+Rc-IPG-phosphonate
1r50 - BsLip+Sc-IPG-phosphonate
1k8q - Lip+phosphonate – dog
1ex9 – Lip+Rc-(Rp,Sp)-1,2-dioctylcarbamoyl-glycero-3-O-phosphonate – Pseudomonas aeruginosa
5tgl – RmLip+N-hexyl-phosphonate
1lpb – Lip (pig)+colipase+C11 alkyl phosphonate
3icw – CaLipB (mutant) +methyl hydrogen R hexylphosphonate
3a70 – PsLip+diethyl phosphate
Lipase conjugated with analogs to its reaction intermediates
1lpn, 1lpo, 1lpp – CrLip+ sulfonates
3rar – CrLip+ phosphonate
1qz3 – EaEst2(mutant) (LIPE)+hexadecanesulfonate
Lipase showing bile-salt binding site
1aql – cBSSL+taurocholate
Lipase with substrate bound at active site
2zyh – AfLip (mutant)+fatty acid – Archaeoglobus fulgidus
2zyi - AfLip+fatty acid+Ca
2zyr - AfLip+fatty acid+Mg
2zys - AfLip+fatty acid+Cl
1gt6 – TlLip+oleic acid - lipid ligand
Lipase conjugated to transition-state analogs showing the binding mode of the enzyme catalysis
1ys1 – BhLip+hexylphosphonic acid (R) 2-methyl-3-phenylpropyl ester
1ys2 – BhLip+hexylphosphonic acid (S) 2-methyl-3-phenylpropyl ester
1hqd – Lip+1-phenoxy-2-acrtoxy butane – Pseudomonas cepacia
Lipase+lipase chaperone
2es4 – Lip+lipase chaperone C-terminal - Burkholderia glumae
References
- ↑ [1] 1HPL PDB SUM
- ↑ [2] A cross-linked complex between horse pancreatic lipase and colipase
- ↑ [3] History of Lipids
- ↑ [4] 1HPL PDB
- ↑ http://www.pdb.org/pdb/explore/explore.do?structureId=1HPL
- ↑ http://www.pdb.org/pdb/explore/remediatedSequence.do?structureId=1HPL
- ↑ http://www.springerlink.com/content/g5h1613440115701/fulltext.pdf
- ↑ Fundamentals of Biochemistry...
- ↑ Thomas, A. etc. "Role of the Lid Hydrophobicity Pattern in Pancreatic Lipase Activity", The Journal of Biological Chemistry, 2005 September 22; 270 (48): 40074-40083.
- ↑ Fundamentals of Biochemistry...
- ↑ Crandall,W., Lowe, M. "Colipase Residues Glu64 and Arg65 Are Essential for Normal Lipase-mediated Fat Digestion in the Presence of Bile Salt Micelles" Journal of Biological Chemistry, 2001, (276) 12505-12512
- ↑ "Colipase". Wikipedia: The Free Encyclopedia. 5 July 2011 [5]
- ↑ "Colipase Residues..."
- ↑ van Tilbeurgh H, etc."Structure of the pancreatic lipase-procolipase complex", 1992 Sep 10;359(6391):159-62. PMID:1522902.[6]
- ↑ http://www.pdb.org/pdb/explore/explore.do?structureId=1ETH
- ↑ http://www.nature.com/nature/journal/v362/n6423/abs/362814a0.html
- ↑ Sussman JL, Harel M, Frolow F, Oefner C, Goldman A, Toker L, Silman I. Atomic structure of acetylcholinesterase from Torpedo californica: a prototypic acetylcholine-binding protein. Science. 1991 Aug 23;253(5022):872-9. PMID:1678899
- ↑ Ollis DL, Cheah E, Cygler M, Dijkstra B, Frolow F, Franken SM, Harel M, Remington SJ, Silman I, Schrag J, et al.. The alpha/beta hydrolase fold. Protein Eng. 1992 Apr;5(3):197-211. PMID:1409539
- ↑ Bourne Y, Martinez C, Kerfelec B, Lombardo D, Chapus C, Cambillau C. Horse pancreatic lipase. The crystal structure refined at 2.3 A resolution. J Mol Biol. 1994 May 20;238(5):709-32. PMID:8182745 doi:http://dx.doi.org/10.1006/jmbi.1994.1331
- ↑ [7] 1LPB PDB SUM
- ↑ "Pancreatic lipase". Wikipedia: The Free Encyclopedia. 7 Nov 2011 [8]
- ↑ Kordik, C., Reitz, A. "Pharmacological Treatment of Obesity: Therapeutic Strategies" Journal of Medicinal Chemistry, 1999 (42).
Proteopedia Page Contributors and Editors (what is this?)
Michal Harel, Quinn R. Murray, Natalie Ziegler, Stephanie Schell, David Canner, Alexander Berchansky, Katelyn Clark, Eric Martz, Leben Tadesse, Joel L. Sussman, Eran Hodis