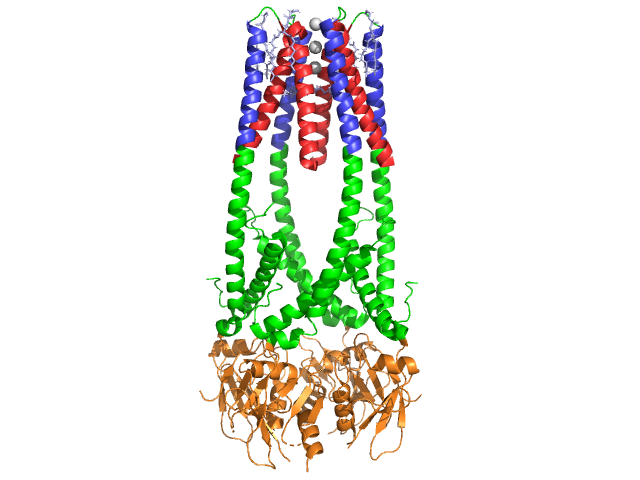
Lipase
From Proteopedia
| Line 14: | Line 14: | ||
== '''Colipase Coenzyme''' == | == '''Colipase Coenzyme''' == | ||
| - | Lipase is activated by the coenzyme colipase, which binds to the C-terminal non-catalytic domain of lipase. Upon binding, active lipase is stabilized for the hydrophobic interaction with triacylglycerides <ref>Fundamentals of Biochemistry...</ref>. Colipase must be present for activation of lipase and acts as a bridge between lipase and the lipid. Without colipase present, the accumulation of amphiphiles at the oil/water interface in the duodenum would prevent pancreatic lipase from binding. <ref>Crandall,W., Lowe, M. "Colipase Residues Glu64 and Arg65 Are Essential for Normal Lipase-mediated Fat Digestion in the Presence of Bile Salt Micelles" Journal of Biological Chemistry, 2001, (276) 12505-12512</ref>. Colipase is | + | Lipase is activated by the coenzyme colipase, which binds to the C-terminal non-catalytic domain of lipase. Upon binding, active lipase is stabilized for the hydrophobic interaction with triacylglycerides <ref>Fundamentals of Biochemistry...</ref>. Colipase must be present for activation of lipase and acts as a bridge between lipase and the lipid. Without colipase present, the accumulation of amphiphiles at the oil/water interface in the duodenum would prevent pancreatic lipase from binding. <ref>Crandall,W., Lowe, M. "Colipase Residues Glu64 and Arg65 Are Essential for Normal Lipase-mediated Fat Digestion in the Presence of Bile Salt Micelles" Journal of Biological Chemistry, 2001, (276) 12505-12512</ref>. Colipase is 10 kDa protein, secreted by the pancreas in its inactive form, which must be activated by trypsin before interacting with lipase. Colipase is a small protein cofactor with 5 conserved <scene name='Lipase/Colipase/3'>disulfide bonds</scene> (shown in yellow) <ref>"Colipase". Wikipedia: The Free Encyclopedia. 5 July 2011 [http://en.wikipedia.org/wiki/Colipase]</ref>, and 2 <scene name='Lipase/Colipase/4'>surfaces</scene>- a hydrophilic surface (site of lipase C-terminal interaction- shown in blue) and a hydrophobic surface (contains multiple hydrophobic loops to bridge the lipid- shown in white)<ref>"Colipase Residues..."</ref>. Colipase and lipase <scene name='Lipase/Contacts/2'>contacts</scene>are opposite of the active site on the C-terminal (contacts are regions of pink and yellow, with water molecules shown in darker blue). The enzymes are bound by polar interactions such as <scene name='Lipase/Salt_bridges/2'>salt bridges</scene>, <scene name='Lipase/Hphobic_interactions/1'>hydrophobic interactions</scene> and <scene name='Lipase/Hydrogen_bonds_non_water/1'>hydrogen bonds</scene> <ref>van Tilbeurgh H, etc."Structure of the pancreatic lipase-procolipase complex", 1992 Sep 10;359(6391):159-62. PMID:1522902.[http://www.proteopedia.org/wiki/index.php/1n8s]</ref>. |
| Line 21: | Line 21: | ||
== '''Lipase Catalytic Mechanism''' == | == '''Lipase Catalytic Mechanism''' == | ||
| - | Lipase activation at the lipid-water interface of triacylglycerides, in the presence of colipase and bile salts, is known as interfacial activation. For the hydroloysis reaction to take place, colipase anchors lipase to the lipid-water membrane of the micelle and a surface change occurs on lipase. Colipase's 4 hydrophobic loops interact with the hydrophobic atmosphere of the triacylglyceride initiating the lipase active site binding to the lipid, and lid opening to reveal a more hydrophobic environment for the triacylglycerol. | + | Lipase activation at the lipid-water interface of triacylglycerides, in the presence of colipase and bile salts, is known as interfacial activation. For the hydroloysis reaction to take place, colipase anchors lipase to the lipid-water membrane of the micelle and a surface change occurs on lipase. Colipase's 4 hydrophobic loops interact with the hydrophobic atmosphere of the triacylglyceride initiating the lipase active site binding to the lipid, and lid opening to reveal a more hydrophobic environment for the triacylglycerol. This allows the triacylglycerol to interact with key active site residues, specifically the catalytic triad. A diverse array of lipase enzymes are found in nature, occupying diverse protein scaffolds, but most are built upon an alpha/beta hydrolase fold<ref>PMID: 1678899</ref><ref>PMID:1409539 </ref> and possess a [[chymotrypsin]]-like <scene name='Lipase/Catalytic_site_outerview/1'>catalytic triad </scene>comprised of an acidic residue, a histidine, and a serine nucleophile. In the case of horse pancreatic lipase, the catalytic triad is comprised of <scene name='Lipase/Catalytic_triad/4'>Ser 152, Asp 176 and His 263. </scene><ref>PMID:8182745</ref>. This catalytic triad functions like most found in nature, first with the aspartic acid forming a hydrogen bond with His 263, increasing the pKa of the histidine imidazole nitrogen. This allows the histidine to act as a powerful general base and deprotonate the serine. The deprotonated serine then can serve as a nucleophile and attack the ester carbonyl of one of the fatty acids on the 1 or 3 carbons of the glycerol backbone of the lipid substrate. Upon attacking the lipid, a negatively charged tetrahedral intermediate is formed (Reaction 1). It is stabilized in the oxyanion hole by two residues: <scene name='Lipase/Catalytic_triad_with_oxyanion/2'>Phe 77 and Leu 153</scene>. |
[[Image:M0218.stg01.gif]] | [[Image:M0218.stg01.gif]] | ||
| Line 41: | Line 41: | ||
== '''Protein - Substrate Interactions''' == | == '''Protein - Substrate Interactions''' == | ||
Lipase binds <scene name='Lipase/Substrate_contacts/1'>substrates such as cholesteryl linoleate</scene> with numerous hydrophobic contacts. As is seen here the lipase interacts with the alkyl group of cholesteryl linoleate via a hydrophobic rift within the protein. This rift orients the molecule to optimize the lipolysis reaction. | Lipase binds <scene name='Lipase/Substrate_contacts/1'>substrates such as cholesteryl linoleate</scene> with numerous hydrophobic contacts. As is seen here the lipase interacts with the alkyl group of cholesteryl linoleate via a hydrophobic rift within the protein. This rift orients the molecule to optimize the lipolysis reaction. | ||
| - | Shown in this scene is lipase from the yeast ''Candida rugosa'' in <scene name='Lipase/Complex_2/1'>complex</scene> with two molecules of cholesteryl linoleate (grey). The active site residues including | + | Shown in this scene is lipase from the yeast ''Candida rugosa'' in <scene name='Lipase/Complex_2/1'>complex</scene> with two molecules of cholesteryl linoleate (grey). The active site residues including Ser152, Asp176, and His263 are shown in red stick representation. Lipase can accommodate two lipid molecules due to the fact that it's two identical subunits catalyze an identical reaction. One lipase molecule can catalyze two lipolysis reactions at a time. |
Revision as of 02:56, 13 April 2012
| |||||||||||
3D Structures of Lipase
Update November 2011
Eukaryote natives:
1hpl – hLip – horse
1hlg – hLip – human - gastric
3jw8, 3hju – mono-glyceride hLip
1jmy – hBSSL
1akn – cBSSL – cattle
2bce - cBSSL (mutant)
1f6w - cBSSL – catalytic domain
3o0d – Lip – Yarrowia lipolytica
1gpl – Lip – Guinea pig
Prokaryote natives:
3guu, 1lbs, 1lbt, 1tca, 1tcb, 1tcc – CaLipA – Candida antarctica
2veo – CaLipA – closed state
3icv – CaLipB (mutant)
1gz7, 1lpm, 1lps– CrLip 2 – Candida rugosa - closed state
1crl, 1trh – CrLip – open state
1llf – Lip – Candida cylindracea
3g7n – Lip - Penicillium expansum
1tia - Lip – Penicillium camemberti
2qua, 2qub – LipA – Serratia marcescens
2hih – Lip – Staphylococcus hyicus
2fx5 – Lip – Pseudomonas mendocina
1yzf – Lip – Enterococcus faecalis
1dt3, 1dt5, 1dte, 1du4, 1ein, 1tib – TlLip - Thermomyces lanuginose
1jfr – Lip – Streptomyces exfoliates
1oil – BcLip - Burkholderia cepacia
2lip – BcLip – open state
1cvl – Lip – Chromobacterium viscosum
1lgy – Lip II – Rhizopus niveus
1tic - Lip – Rhizopus oryzae
1thg – Lip – Geotrichum candidum
3tgl, 4tgl, 1tgl – RmLip– Rhyzomucor miehei
2zvd – PsLip - Pseudomonas sp. – open state
2z8x - PsLip – extracellular
2zj6, 2zj7 – PsLip (mutant)
2z8z – PsLip(mutant) – closed state
3lip, 3a6z - Lip - Pseudomonas cepacia – open state
1qge, 1tah – Lip – Pseudomonas glumae
2w22 – Lip – Geobacillus thermocatenulatus
1ji3, 1ku0 – Lip – Bacillus stearothermophilus
1ah7 - Lip – Bacillus cereus
2qxt, 2qxu, 1isp, 1i6w - BsLip – Bacillus subtilis
3d2a, 3d2b, 3d2c, 1t2n, 1t4m, 3qmm - BsLip (mutant)
2ory – Lip – Photobacterium lypoliticum
2z5g, 2dsn – Lip T1 – Geobacillus zalihae
3p94 – Lip – Parabacteroides distasonis
3ngm – Lip – Gibberella zeae
Lipase/colipase complexes. The colipase is a co-enzyme whose binding to lipase optimizes the enzymatic activity
1n8s – hLip+colipase II
1eth, 1lpa - Lip+colipase II - pig
Hormone-sensitive-lipases (LIPE) hydrolyze the first fatty acid of the triacylglycerol substrate
3k6k – EstE7(LIPE) – metagenome library
3fak, 3dnm – EstE5(LIPE) – metagenome library
1evq – AaEst2(LIPE) – Alicyclobacillus acidocaldarius
1u4n – AaEst2(LIPE) (mutant)
Putative lipases; Proteins with unknown function but structural similarity to lipase obtained in structural genomics projects.
2rau - Lip – Sulfolobus solfataricus
3bxp, 3d3n - Lip – Lactobacillus plantarum
3e0x - Lip – Clostridium acetobutylicum
1z8h – Lip – Nostoc sp. PCC 712
1vj3 - Lip – Nostoc sp.
3bzw – Lip - Bacteroides thetaiotaomicron
2pbl – Lip - Silicibacter
Lipase + inhibitors
3jwe, 3pe6 - mono-glyceride hLip + SAR629 – covalent inhibitor
3l1h – EstE5(LIPE)+FeCl3 – noninvasive inhibitor
3l1i, 3l1j - EstE5(LIPE)+CuSO4 – noninvasive inhibitor
3lij - EstE5(LIPE)+ZnSO4– noninvasive inhibitor
3h18, 3h17 - EstE5 (LIPE)+PMSF
3h19, 3h1b, 3h1a – EstE5 (LIPE)+methyl alcohol
3h1a – EstE5 SLIPE)+ethyl alcohol
3h19 – EstE5 SLIPE)+isopropyl alcohol
3g9t, 3g9u - EstE5 (HSLIPE)+p-nitrophenyl butyrate
3g9z - EstE5 (LIPE) +p-nitrophenyl caprylate
2nw6 – BcLip+ S inhibitor
4lip, 5lip, 1r4z, 1r50 – BcLip+ Rc-(Rp,Sp)-1,2-dioctylcarbamoyl-glycero-3-O-phosphonate
1r4z – BsLip+Rc-IPG-phosphonate
1r50 - BsLip+Sc-IPG-phosphonate
1k8q - Lip+phosphonate – dog
1ex9 – Lip+Rc-(Rp,Sp)-1,2-dioctylcarbamoyl-glycero-3-O-phosphonate – Pseudomonas aeruginosa
5tgl – RmLip+N-hexyl-phosphonate
1lpb – Lip (pig)+colipase+C11 alkyl phosphonate
3icw – CaLipB (mutant) +methyl hydrogen R hexylphosphonate
3a70 – PsLip+diethyl phosphate
Lipase conjugated with analogs to its reaction intermediates
1lpn, 1lpo, 1lpp – CrLip+ sulfonates
3rar – CrLip+ phosphonate
1qz3 – EaEst2(mutant) (LIPE)+hexadecanesulfonate
Lipase showing bile-salt binding site
1aql – cBSSL+taurocholate
Lipase with substrate bound at active site
2zyh – AfLip (mutant)+fatty acid – Archaeoglobus fulgidus
2zyi - AfLip+fatty acid+Ca
2zyr - AfLip+fatty acid+Mg
2zys - AfLip+fatty acid+Cl
1gt6 – TlLip+oleic acid - lipid ligand
Lipase conjugated to transition-state analogs showing the binding mode of the enzyme catalysis
1ys1 – BhLip+hexylphosphonic acid (R) 2-methyl-3-phenylpropyl ester
1ys2 – BhLip+hexylphosphonic acid (S) 2-methyl-3-phenylpropyl ester
1hqd – Lip+1-phenoxy-2-acrtoxy butane – Pseudomonas cepacia
Lipase+lipase chaperone
2es4 – Lip+lipase chaperone C-terminal - Burkholderia glumae
References
- ↑ [1] 1HPL PDB SUM
- ↑ [2] A cross-linked complex between horse pancreatic lipase and colipase
- ↑ [3] History of Lipids
- ↑ [4] 1HPL PDB
- ↑ http://www.pdb.org/pdb/explore/explore.do?structureId=1HPL
- ↑ http://www.pdb.org/pdb/explore/remediatedSequence.do?structureId=1HPL
- ↑ http://www.springerlink.com/content/g5h1613440115701/fulltext.pdf
- ↑ Fundamentals of Biochemistry...
- ↑ Thomas, A. etc. "Role of the Lid Hydrophobicity Pattern in Pancreatic Lipase Activity", The Journal of Biological Chemistry, 2005 September 22; 270 (48): 40074-40083.
- ↑ Fundamentals of Biochemistry...
- ↑ Crandall,W., Lowe, M. "Colipase Residues Glu64 and Arg65 Are Essential for Normal Lipase-mediated Fat Digestion in the Presence of Bile Salt Micelles" Journal of Biological Chemistry, 2001, (276) 12505-12512
- ↑ "Colipase". Wikipedia: The Free Encyclopedia. 5 July 2011 [5]
- ↑ "Colipase Residues..."
- ↑ van Tilbeurgh H, etc."Structure of the pancreatic lipase-procolipase complex", 1992 Sep 10;359(6391):159-62. PMID:1522902.[6]
- ↑ http://www.pdb.org/pdb/explore/explore.do?structureId=1ETH
- ↑ http://www.nature.com/nature/journal/v362/n6423/abs/362814a0.html
- ↑ Sussman JL, Harel M, Frolow F, Oefner C, Goldman A, Toker L, Silman I. Atomic structure of acetylcholinesterase from Torpedo californica: a prototypic acetylcholine-binding protein. Science. 1991 Aug 23;253(5022):872-9. PMID:1678899
- ↑ Ollis DL, Cheah E, Cygler M, Dijkstra B, Frolow F, Franken SM, Harel M, Remington SJ, Silman I, Schrag J, et al.. The alpha/beta hydrolase fold. Protein Eng. 1992 Apr;5(3):197-211. PMID:1409539
- ↑ Bourne Y, Martinez C, Kerfelec B, Lombardo D, Chapus C, Cambillau C. Horse pancreatic lipase. The crystal structure refined at 2.3 A resolution. J Mol Biol. 1994 May 20;238(5):709-32. PMID:8182745 doi:http://dx.doi.org/10.1006/jmbi.1994.1331
- ↑ [7] 1LPB PDB SUM
- ↑ "Pancreatic lipase". Wikipedia: The Free Encyclopedia. 7 Nov 2011 [8]
- ↑ Kordik, C., Reitz, A. "Pharmacological Treatment of Obesity: Therapeutic Strategies" Journal of Medicinal Chemistry, 1999 (42).
Proteopedia Page Contributors and Editors (what is this?)
Michal Harel, Quinn R. Murray, Natalie Ziegler, Stephanie Schell, David Canner, Alexander Berchansky, Katelyn Clark, Eric Martz, Leben Tadesse, Joel L. Sussman, Eran Hodis