Sandbox Reserved 489
From Proteopedia
| Line 25: | Line 25: | ||
<Structure load='2ren' size='400' frame='true' align='right' caption='Mature Renin' scene='Insert optional scene name here' /> | <Structure load='2ren' size='400' frame='true' align='right' caption='Mature Renin' scene='Insert optional scene name here' /> | ||
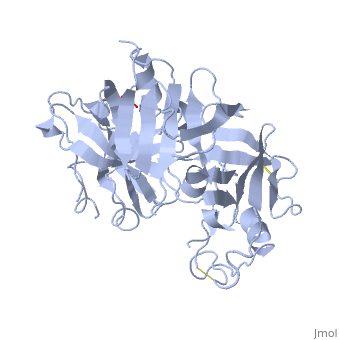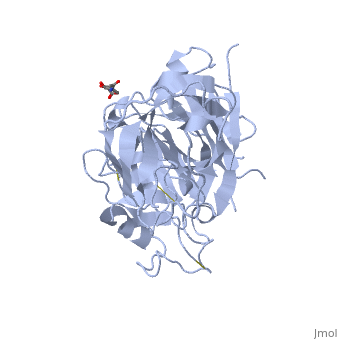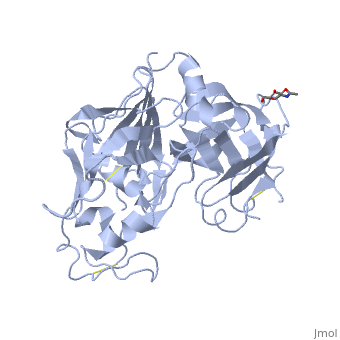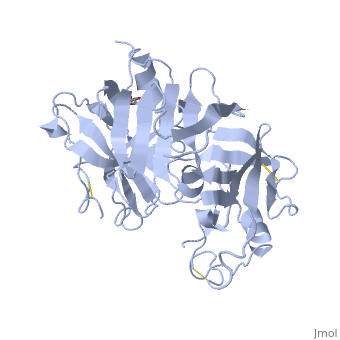
| - | The precursor of renin is a 406 amino acid residue protein. <scene name='Sandbox_Reserved_489/Signal_domain/1'>Residues 1-23</scene> are a signal peptide sequence and residues 24-66 are cleaved to produce the mature 340 amino acid residue <scene name='Sandbox_Reserved_489/Mature_renin/1'>mature renin</scene>. The secondary structural elements of renin include <scene name='Sandbox_Reserved_489/Betasheetscolors/1'>29 antiparallel beta sheets</scene>, <scene name='Sandbox_Reserved_489/Betabridges/1'>3 beta bridges</scene>, <scene name='Sandbox_Reserved_489/Alphahelixes/1'>4 alpha helices</scene>, <scene name='Sandbox_Reserved_489/310heleices/1'>2 3-10 helices</scene>, and <scene name='Sandbox_Reserved_489/Turns/1'>18 turns</scene>. The most impressive structural feature of renin is the <scene name='Sandbox_Reserved_489/Betasheetspiral/1'>antiparallel beta sheet elongated spiral barrel</scene>. <scene name='Sandbox_Reserved_489/Hydrophobichydrophillic/1'>Hydrophilic and hydrophobic residues</scene> are practically evenly distributed throughout renin. The alternating hydrophilic and hydrophobic residues most likely assist in folding of the unique spiral structure. The active site of renin contains two essential <scene name='Sandbox_Reserved_489/Activesiteasps2/2'>aspartate residues</scene>. | + | The precursor of renin is a 406 amino acid residue protein. <scene name='Sandbox_Reserved_489/Signal_domain/1'>Residues 1-23</scene> are a signal peptide sequence and residues 24-66 are cleaved to produce the mature 340 amino acid residue <scene name='Sandbox_Reserved_489/Mature_renin/1'>mature renin</scene>. The secondary structural elements of renin include <scene name='Sandbox_Reserved_489/Betasheetscolors/1'>29 antiparallel beta sheets</scene>, <scene name='Sandbox_Reserved_489/Betabridges/1'>3 beta bridges</scene>, <scene name='Sandbox_Reserved_489/Alphahelixes/1'>4 alpha helices</scene>, <scene name='Sandbox_Reserved_489/310heleices/1'>2 3-10 helices</scene>, and <scene name='Sandbox_Reserved_489/Turns/1'>18 turns</scene>. The most impressive structural feature of renin is the <scene name='Sandbox_Reserved_489/Betasheetspiral/1'>antiparallel beta sheet elongated spiral barrel</scene>. <scene name='Sandbox_Reserved_489/Hydrophobichydrophillic/1'>Hydrophilic and hydrophobic residues</scene> are practically evenly distributed throughout renin. The alternating hydrophilic and hydrophobic residues most likely assist in folding of the unique spiral structure. The active site of renin contains two essential <scene name='Sandbox_Reserved_489/Activesiteasps2/2'>aspartate residues</scene>. There are <scene name='Sandbox_Reserved_489/Catalyticmotifs/1'>2 catalytic motifs</scene> after each of the two aspartate residues. |
Post translational modifications of renin include; precursor cleavage of propetide to produce active mature renin, disulfide bond formation, and glycosylation of certain residues. Disulfide bonds are form to connect serine residues <scene name='Sandbox_Reserved_489/Disulfidebond1/1'>51 to 58</scene>, <scene name='Sandbox_Reserved_489/Disulfidebond2/1'>217 to 221</scene>, and <scene name='Sandbox_Reserved_489/Disulfidebond3/1'>259 to 296</scene>. <scene name='Sandbox_Reserved_489/Glycosylated/1'>Two asparagine residues</scene> at positions 15 and 75 are glycosylated in the mature and active renin. | Post translational modifications of renin include; precursor cleavage of propetide to produce active mature renin, disulfide bond formation, and glycosylation of certain residues. Disulfide bonds are form to connect serine residues <scene name='Sandbox_Reserved_489/Disulfidebond1/1'>51 to 58</scene>, <scene name='Sandbox_Reserved_489/Disulfidebond2/1'>217 to 221</scene>, and <scene name='Sandbox_Reserved_489/Disulfidebond3/1'>259 to 296</scene>. <scene name='Sandbox_Reserved_489/Glycosylated/1'>Two asparagine residues</scene> at positions 15 and 75 are glycosylated in the mature and active renin. | ||
Revision as of 21:26, 14 April 2012
| This Sandbox is Reserved from 13/03/2012, through 01/06/2012 for use in the course "Proteins and Molecular Mechanisms" taught by Robert B. Rose at the North Carolina State University, Raleigh, NC USA. This reservation includes Sandbox Reserved 451 through Sandbox Reserved 500. | ||||||
To get started:
More help: Help:Editing For more help, look at this link: http://www.proteopedia.org/wiki/index.php/Help:Getting_Started_in_Proteopedia Renin Renin, also known as angiotensinogenase, is an aspartyl protease and belongs to the protein family peptidase A1. Aspartyl proteases are endopeptidases that typically use two aspartate residues in the active site in a reduction-oxidation reaction with water to specifically cleave peptide substrates. Mature renin circulates in the blood and contains 340 amino acid residues and has a mass of approximately 37 kDa. The function of renin is to cleave the angiotensin I precursor, angiotensinogen, to produce angiotensin I. Renin is secreted by the kidneys. The kidneys act both directly and indirectly to regulate arterial blood pressure and provide the major long term mechanism of blood pressure and control. The direct mechanism changes blood volume independently of hormones. When blood pressure and blood volume increase the kidneys can not filter all of the liquids and thus liquids are lost in the urine to decrease blood pressure and blood volume. The indirect mechanism, or the renin-angiotensin system (RAS), controls blood volume and blood pressure through renin and two forms of angiotensin. Renin is involved in the rate limiting first step of a cascade that eventually produces angiotensin II. The specialized granular cells of the juxtaglomerular apparatus secrete renin when stimulated by the macula densa when blood pressure or blood volume decreases. Renin circulating in the blood stream cleaves a small 10 residue portion of plasma protein angiotensinogen that is secreted by the liver. Cleavage of angiotensinogen produces the inactive precursor angiotensin I that is converted to angiotensin II by angiotensin-converting enzyme primarily in the lungs. Angiotensin II increases blood pressure in three ways.
Additionally, angiotensin II triggers the sensation of thirst. The release of renin into the blood stream ultimately raises blood pressure. The kidneys restore and maintain blood pressure homeostasis by regulating blood volume through the action of renin. Although blood volume varies with age, body size, and sex, renal mechanisms usually maintain it to 5 liters. Renin has been identified in many eukaryotic organisms including; humans, mice, marmosets, monkeys, chimpanzees, macaques, dogs, rats, frogs, and zebrafish. The function of renin in all organisms is similar, but the sequence and peptide length vary slightly. Renin is important clinically because renin inhibitors, such as Aliskiren can be used to treat hypertension. Structure
The precursor of renin is a 406 amino acid residue protein. are a signal peptide sequence and residues 24-66 are cleaved to produce the mature 340 amino acid residue . The secondary structural elements of renin include , , , , and . The most impressive structural feature of renin is the . are practically evenly distributed throughout renin. The alternating hydrophilic and hydrophobic residues most likely assist in folding of the unique spiral structure. The active site of renin contains two essential . There are after each of the two aspartate residues. Post translational modifications of renin include; precursor cleavage of propetide to produce active mature renin, disulfide bond formation, and glycosylation of certain residues. Disulfide bonds are form to connect serine residues , , and . at positions 15 and 75 are glycosylated in the mature and active renin. FunctionRenin secretion is stimulated by a decrease in arterial blood pressure, a decrease in sodium chloride levels in kidney nephrons, or sympathetic nervous system activity. The substrate of renin, angiotensinogen, is a 452 amino acid residue in humans. Renin utilizes two aspartate residues in the to cleave the peptide bond between leucine and valine residues on angiotensinogen. Angiotensin I is an inactive short peptide of 10 amino acids that is produced by the renin cleavage reaction. The two aspartate residues operate most efficiently at acidic pH because one of the carbonyl groups must be deprotonated to accept a proton from water. The mechanism of the catalysis is an acid base transfer of water between the two aspartate residues. Initially one aspartate residue carbonyl is deprotonated and the other is protonated. The deprotonated aspartate removes a proton from water allowing the water to attack the carbonyl of the beptide bond in the substrate forming a tetrahedral oxyanion intermediate on the substrate. Rearrangement of the intermediate causes protonation of the amide on the substrate completing the cleavage reaction. Renin can also bind the renin receptor ATPase H(+)-transporting lysosomal accessory protein 2 (ATP6AP2) to convert angiotensinogen to angiotensin I at a much greater rate. |