User:Mumal Usman/Sandbox 1
From Proteopedia
| Line 28: | Line 28: | ||
1. Lang and Phillip 1999 | 1. Lang and Phillip 1999 | ||
| - | 2. Embers 2007 | + | 2. Embers ME, Liang FT, Howell JK, Jacobs MB, Purcell JE, Norris SJ, Johnson BJ, Philipp MT. 2007. Antigenicity and recombination of VlsE, the antigenic variation protein of Borrelia burgdorferi, in rabbits, a host putatively resistant to long-term infection with this spirochete. ''FEMS Immunol Med Microbiol''. 50(3):421-9. [http://www.ncbi.nlm.nih.gov/pubmed/17596185 PMID: |
| + | 17596185] | ||
| - | 3. Eicken | + | 3. Eicken C, Sharma V, Klabunde T, Lawrenz MB, Hardham JM, Norris SJ, Sacchettini JC. 2002. Crystal structure of Lyme disease variable surface antigen VlsE of Borrelia burgdorferi. ''J Biol Chem.'' 277(24):21691-6. [http://www.ncbi.nlm.nih.gov/pubmed/11923306 PMID: 11923306] |
4. Zhang et al. 1997 | 4. Zhang et al. 1997 | ||
| Line 38: | Line 39: | ||
6. Eicken C, Sharma V, Klabunde T, Lawrenz MB, Hardham JM, Norris SJ, Sacchettini JC. 2002. Crystal structure of Lyme disease variable surface antigen VlsE of Borrelia burgdorferi. ''J Biol Chem.'' 277(24):21691-6.[http://www.ncbi.nlm.nih.gov/pubmed/11923306 PMID: 11923306] | 6. Eicken C, Sharma V, Klabunde T, Lawrenz MB, Hardham JM, Norris SJ, Sacchettini JC. 2002. Crystal structure of Lyme disease variable surface antigen VlsE of Borrelia burgdorferi. ''J Biol Chem.'' 277(24):21691-6.[http://www.ncbi.nlm.nih.gov/pubmed/11923306 PMID: 11923306] | ||
| - | 7. Coutte L, Botkin DJ, Gao L, Norris SJ. 2009. Detailed analysis of sequence changes occurring during vlsE antigenic variation in the mouse model of Borrelia burgdorferi infection. PLoS Pathog. Feb;5(2):e1000293. [http://www.ncbi.nlm.nih.gov/pubmed/19214205 PMID: 19214205] | + | 7. Coutte L, Botkin DJ, Gao L, Norris SJ. 2009. Detailed analysis of sequence changes occurring during vlsE antigenic variation in the mouse model of Borrelia burgdorferi infection. ''PLoS Pathog''. Feb;5(2):e1000293. [http://www.ncbi.nlm.nih.gov/pubmed/19214205 PMID: 19214205] |
Revision as of 07:35, 30 April 2012
Contents |
Introduction
|
The successful infection of Lyme disease can be attributed to its many membrane proteins. The surface lipoprotein, , is responsible for the infectious properties of the Lyme disease spirochete, Borrelia burgdorferi[1]. VlsE uses several immune evasive strategies, giving the disease its characteristically infectious behavior. Specifically, antigenic variation and suppression of innate and acquired immune response aid the disease in remaining in the host[2]. Antigenic variation is the most efficient strategy in order to protect the infectious properties of the spirochete[3]. Expressed antigenic variation is produced by segmental gene conversation through variable regions of the lipoprotein[4,5].
Structure
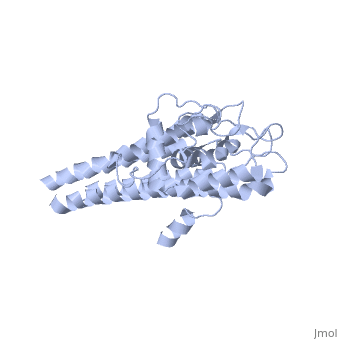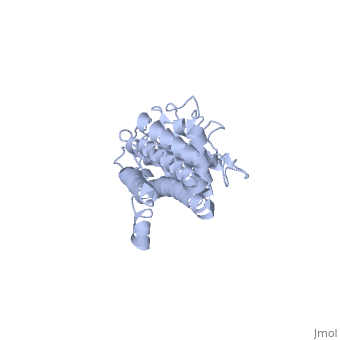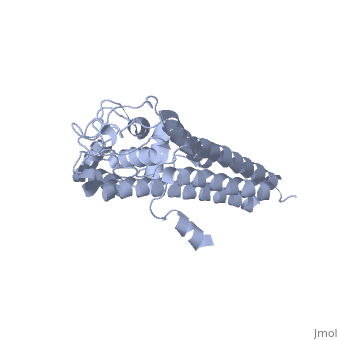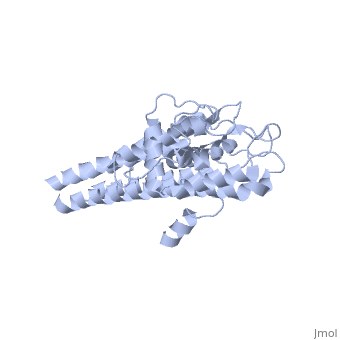
Approximately 75% of the VlsE protein primary structure is invariable and highly conserved[3]. There are N and C terminal regions of invariability, or direct repeats, which flank the cassette region where the invariable regions of the cassette reside[3], . Within the cassette region exists silent cassettes that are unexpressed. Assuring the survival of the invariable regions is of utmost importance for proper structure and physiological function of the protein. By encasing the invariable regions within the distal core of the protein, the variable regions protect the invariable regions from the host immune system.
The protein is made up of eleven alpha(α) helices, seven of which (α4-α10) reside in the core region of the distal portion of the protein[3]. These α-helices make up the cassette region of the protein, within which there are six variable regions (VR) and (IR)[1,3] . The six variable regions provide the variation necessary to evade the host immune systemi. Variability is produced via amino acid substitutions concentrated to a small portion of the variable region, which likely produces a conformational change of the epitope allowing it to elude the host immune system[3].
Although the exact function of the VlsE surface lipoprotein is unknown, three invariable regions are of particular interest, [1,3]. IR6 forms alpha helix 10, which is almost entirely buried within the center of the membrane distal regions encased by the variable regions, it has only 13.7% surface exposure[3] (GREEN LINK). IR2 and IR4 were also found to have significant antigenicity, however only in mice and at much lower rates when compared to IR6[1]. By encasing these invariable regions within the distal core of the protein, the variable regions provide protection for the invariable regions from the host immune system.
Functionality
Although the exact function of the VlsE surface lipoprotein is unknown, three invariable regions are of particular interest, IR6, IR2, and IR4 [1,3]. IR6 forms alpha helix 10, which is almost entirely buried within the center of the membrane distal regions encased by the variable regions, it has only 13.7% [3]. IR2 and IR4 were also found to have significant antigenicity, however only in mice and at much lower rates when compared to IR6 [1]. By encasing these invariable regions within the distal core of the protein, the variable regions provide protection for the invariable regions from the host immune system.
Antigenic Variation
When mice were infected segments of silent cassette recombine into VlsE cassette region through a yet unknown gene conversion mechanism, the silent cassette region is inserted, but remains unaltered otherwise[1,4]. Antigenic variation in VlsE involves the vls locus of b. burgdorferi on the linear plasmid Ip 28-1. The locus contains both the expression site of the VlsE lipoprotein and 15 contiguous silent cassette region six. These silent cassettes are closely homologous to the central cassette regions; the only differences being from the six variable regions within each cassette. The variation usually begins 4 days after infection and continues throughout the course of the infection[6].
References
1. Lang and Phillip 1999
2. Embers ME, Liang FT, Howell JK, Jacobs MB, Purcell JE, Norris SJ, Johnson BJ, Philipp MT. 2007. Antigenicity and recombination of VlsE, the antigenic variation protein of Borrelia burgdorferi, in rabbits, a host putatively resistant to long-term infection with this spirochete. FEMS Immunol Med Microbiol. 50(3):421-9. [http://www.ncbi.nlm.nih.gov/pubmed/17596185 PMID:
17596185]
3. Eicken C, Sharma V, Klabunde T, Lawrenz MB, Hardham JM, Norris SJ, Sacchettini JC. 2002. Crystal structure of Lyme disease variable surface antigen VlsE of Borrelia burgdorferi. J Biol Chem. 277(24):21691-6. PMID: 11923306
4. Zhang et al. 1997
5. Zhang JR, Norris SJ. 1998. Genetic variation of the Borrelia burgdorferi gene vlsE involves cassette-specific, segmental gene conversion. Infect Immun. 8:66(8):3698-704.PMID: 9673251
6. Eicken C, Sharma V, Klabunde T, Lawrenz MB, Hardham JM, Norris SJ, Sacchettini JC. 2002. Crystal structure of Lyme disease variable surface antigen VlsE of Borrelia burgdorferi. J Biol Chem. 277(24):21691-6.PMID: 11923306
7. Coutte L, Botkin DJ, Gao L, Norris SJ. 2009. Detailed analysis of sequence changes occurring during vlsE antigenic variation in the mouse model of Borrelia burgdorferi infection. PLoS Pathog. Feb;5(2):e1000293. PMID: 19214205