Sandbox Reserved 496
From Proteopedia
| Line 18: | Line 18: | ||
| + | {{Clear}} | ||
==='''Mechanism of Action'''=== | ==='''Mechanism of Action'''=== | ||
---- | ---- | ||
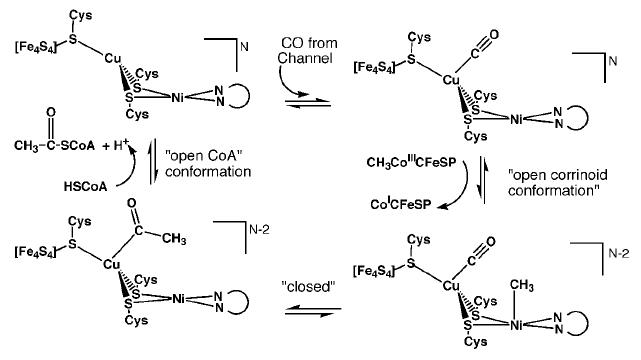
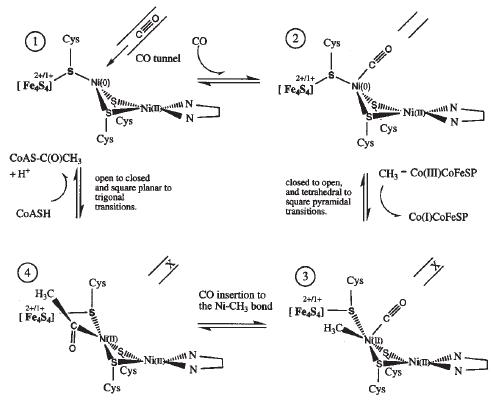
| - | There are two proposed mechanisms for the catalytic action of the A-cluster. | + | There are two proposed mechanisms for the catalytic action of the A-cluster <ref name="Cu"/> <ref name="Zn">PMID:12627225</ref>. |
[[Image:A_cluster_1.JPG|frame|Proposed mechanism for ACS activity with Cu-Ni ions in the binuclear site of the A-cluster.]] [[Image:A_cluster_2.JPG|frame|Proposed mechanism for ACS activity with Ni-Ni ions in the binuclear site of the A-cluster.]] | [[Image:A_cluster_1.JPG|frame|Proposed mechanism for ACS activity with Cu-Ni ions in the binuclear site of the A-cluster.]] [[Image:A_cluster_2.JPG|frame|Proposed mechanism for ACS activity with Ni-Ni ions in the binuclear site of the A-cluster.]] | ||
| - | Proposed catalytic action of the C-cluster. | + | {{Clear}} |
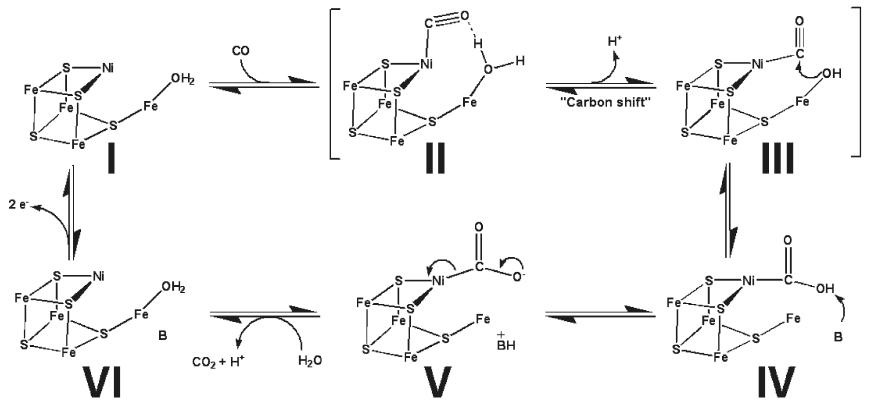
| + | Proposed catalytic action of the C-cluster <ref name="CN">PMID:19583207</ref>. | ||
[[Image:C_cluster.JPG|frame|Proposed mechanism for CODH activity in the C-cluster.]] | [[Image:C_cluster.JPG|frame|Proposed mechanism for CODH activity in the C-cluster.]] | ||
| + | {{Clear}} | ||
==='''Possible Applications'''=== | ==='''Possible Applications'''=== | ||
---- | ---- | ||
Revision as of 01:40, 2 May 2012
|Bold text
| This Sandbox is Reserved from 13/03/2012, through 01/06/2012 for use in the course "Proteins and Molecular Mechanisms" taught by Robert B. Rose at the North Carolina State University, Raleigh, NC USA. This reservation includes Sandbox Reserved 451 through Sandbox Reserved 500. | |||||||
To get started:
More help: Help:Editing For more help, look at this link: http://www.proteopedia.org/wiki/index.php/Help:Getting_Started_in_Proteopedia
Bifunctional Carbon Monoxide Dehydrogenase/Acetyl-CoA Synthase (CODH/ACS)
IntroductionPDB codes for the M. thermoacetica enzyme are: 1MJG [1] (shown at right), 1OAO [2], 2Z8Y [3], 3I01 [4], and 3I04 [5]. StructureThe CODH/ACS enzyme from M. thermoacetica is an α2β2 tetramer. Each β subunit (residues 2 to 674) carries out CODH activity, while each α subunit (residues 2 to 729) is responsible for ACS activity. The β subunit has 57% helical and 9% β-sheet character with 31 helices and 15 β-strands. The α subunit is comprised of three domains, two with α+β folds and a third with a helical region at the NH2-terminus of a Rossmann fold which is similar to a portion of the β subunit structure [6]. Overall, the α subunit has 50% helical and 14% β-sheet character with 36 helices and 22 β-strands.
Mechanism of ActionThere are two proposed mechanisms for the catalytic action of the A-cluster [6] [7]. Proposed catalytic action of the C-cluster [8]. Possible Applications
References
|