Sandbox Reserved 496
From Proteopedia
| Line 1: | Line 1: | ||
| - | |'''Bold text'''<!-- PLEASE DO NOT DELETE THIS TEMPLATE --> | + | |'''Bold text''' |
| + | |||
| + | '''DO NOT DELETE UNTIL AFTER JULY 1, 2012.''' | ||
| + | <!-- PLEASE DO NOT DELETE THIS TEMPLATE --> | ||
{{Sandbox_Reserved_Robert_B_Rose_1}} | {{Sandbox_Reserved_Robert_B_Rose_1}} | ||
<!-- PLEASE ADD YOUR CONTENT BELOW HERE --> | <!-- PLEASE ADD YOUR CONTENT BELOW HERE --> | ||
| - | |||
| - | |||
== '''Bifunctional Carbon Monoxide Dehydrogenase/Acetyl-CoA Synthase (CODH/ACS)''' == | == '''Bifunctional Carbon Monoxide Dehydrogenase/Acetyl-CoA Synthase (CODH/ACS)''' == | ||
<Structure load='1mjg' size='400' frame='true' align='right' caption='Carbon monoxide dehydrogenase/acetyl-CoA synthase asymmetric unit containing two α2β2 tetramers.' scene='Insert optional scene name here' /> | <Structure load='1mjg' size='400' frame='true' align='right' caption='Carbon monoxide dehydrogenase/acetyl-CoA synthase asymmetric unit containing two α2β2 tetramers.' scene='Insert optional scene name here' /> | ||
Current revision
|Bold text
DO NOT DELETE UNTIL AFTER JULY 1, 2012.
| This Sandbox is Reserved from 13/03/2012, through 01/06/2012 for use in the course "Proteins and Molecular Mechanisms" taught by Robert B. Rose at the North Carolina State University, Raleigh, NC USA. This reservation includes Sandbox Reserved 451 through Sandbox Reserved 500. | |||||||
To get started:
More help: Help:Editing For more help, look at this link: http://www.proteopedia.org/wiki/index.php/Help:Getting_Started_in_Proteopedia
Bifunctional Carbon Monoxide Dehydrogenase/Acetyl-CoA Synthase (CODH/ACS)
IntroductionCarbon monoxide dehydrogenase/acetyl-CoA synthase (CODH/ACS) is a bifunctional protein which acts as both an oxidoreductase and a transferase by reducing carbon dioxide to carbon monoxide (or the reverse oxidation of carbon monoxide to carbon dioxide) and then catalyzing the synthesis of acetyl-CoA from carbon monoxide, coenzyme A, and the methyl group of an corrinoid iron-sulfur protein. This enzyme plays a key role in the Wood-Ljungdahl pathway which is used by anaerobic, autotrophic bacteria such as Moorella thermoacetica (f. Clostridium thermoaceticum) and Clostridium ljungdahlii for gaseous carbon fixation. End products of the Wood-Ljungdahl pathway include cell biomass, acids (ex. acetate and butyrate), and alcohols (ex. ethanol and butanol) – all of which derive from acetyl-CoA. The Wood-Ljungdahl pathway is also the most energetically favorable carbon fixation pathway, but its use is confined to only obligate, anaerobic species [1]. Since the discovery of the Wood-Ljungdahl pathway in the early 1980’s, significant effort has been put into trying to characterize the substrate binding activity of CODH/ACS with the M. thermoacetica protein used as the model in most case studies [2]. However, little progress was made in defining the exact structure of the protein with crystal structures until the 2000’s. Several reasons why CODH/ACS has received so much attention include the fact that it contains highly disputed metalloclusters, its use of biological organometallic intermediates in reactions, and its contribution to reducing environmental pollutants [3] [4].
StructureThe CODH/ACS enzyme from M. thermoacetica is an with seven metalloclusters. Each 674 residue carries out CODH activity, while each 729 residue is responsible for ACS activity. From the N-terminus to C-terminus, are as follows: an α-helical domain (residues 1-257) followed by two α/β Rossmann-like domains (residues 262-458 and 463-674). The β subunit has 57% helical and 9% β-sheet character with 31 helices and 15 β-strands. The , two with α+β folds and a third with a helical region (residues 1-154) at the N-terminus of a Rossmann (six-stranded α/β) fold (residues 155-316) which is similar to a portion of the β subunit structure [5] [12]. Overall, the α subunit has 50% helical and 14% β-sheet character with 36 helices and 22 β-strands.
Mechanism of Action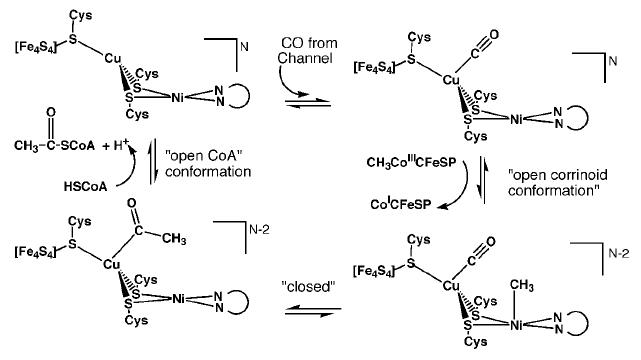 Proposed mechanism 1 for ACS activity with Cu-Ni ions in the binuclear site of the A-cluster. [5] β subunit reactions C-cluster (CODH activity): CO2 + 2H+ + 2e- ↔ CO + H2O B- and D-clusters: electron transfer
A-cluster (ACS activity): CH3-Co(III)-CFeSP + CO + HSCoA ↔ CH3-CO-SCoA + Co(I)-CFeSP + H+
Mechanism 1 suggests that CO created at the C-cluster travels through the hydrophobic tunnel and binds to Cu. Next, the A-cluster switches to an open corrinoid conformation so that the methyl group may be transferred from the corrinoid iron-sulfur protein to the distal Ni ion of the active site. The cluster reverts back to a solvent protected, closed form to generate the acetyl intermediate, and then reopens to allow HSCoA to bind. HSCoA likely binds in the large cavity between the three domains of the α subunit where six Arg residues and Trp418 coincide. Finally, HSCoA is deprotonated and acetylated to form acetyl-CoA [5]. 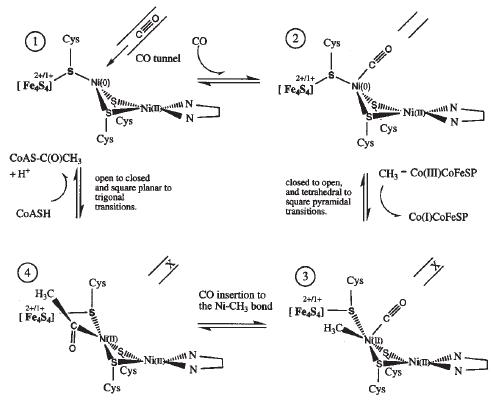 Proposed mechanism 2 for ACS activity with Ni-Ni ions in the binuclear site of the A-cluster. [13]
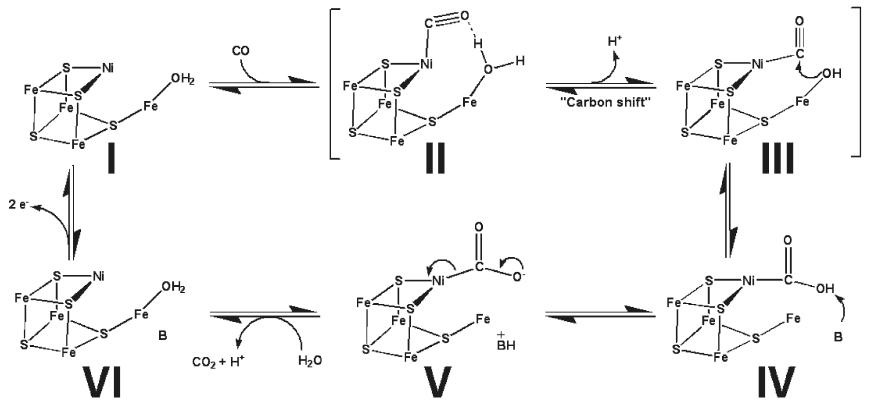 Proposed mechanism for CODH activity in the C-cluster. [2] In the proposed catalytic action of the C-cluster, CO first binds the Ni ion followed by deprotonation of the Fe bound water to yield a reactive hydroxide which promotes nucleophilic attack of CO by the hydroxide. Next, the Ni-COOH intermediate is deprotonated to Ni-COO- which allows release of CO2 as the C-cluster is reduced to a new redox state. Then, electrons are shuttled between the B- and D-clusters to reoxidize the C-cluster to its original redox state [2]. This catalytic cycle is of course operated in reverse to reduce CO2 to CO and allow subsequent production of acetyl-CoA . Possible ApplicationsSince CODH/ACS consumes CO2 it plays a direct part in the reduction of greenhouse gases by converting CO2 into an intermediate which can transformed into more desirable products such as alcohols and acids. A novel application in which CODH/ACS plays a role is the production of bioethanol from renewable natural resources such as wood, grasses, and agricultural residues (ex. corn stover). In this process, the biomass undergoes gasification to yield CO, CO2, and H2. The gases are subsequently fed to bacterial reactor systems containing organisms which utilize the Wood-Ljungdahl pathway and thus produce ethanol. The main goal is to produce a renewable energy source that may be used as an alternative to petroleum. However, at this time, the flux of carbon through the Wood-Ljungdahl pathway does not tend to favor ethanol production so much work remains to be done in this area. On the other hand, the Wood-Ljungdahl pathway does produce significant levels of acetic acid, and to this extent, the pathway is considered a biological equivalent to the Monsanto process for industrial acetic acid production [1]. Roughly 10% of the total biological acetic acid production is attributable to anaerobic, autotrophic bacteria which possess CODH/ACS enzymes. References
|
