Sandbox Reserved 561
From Proteopedia
(New page: <!-- PLEASE DO NOT DELETE THIS TEMPLATE --> {{NCSU_BIOL414_Sandbox}} <!-- PLEASE ADD YOUR CONTENT BELOW HERE --> Hi everyone, Here is your very own Proteopedia Page to play with. This page...) |
|||
| Line 2: | Line 2: | ||
{{NCSU_BIOL414_Sandbox}} | {{NCSU_BIOL414_Sandbox}} | ||
<!-- PLEASE ADD YOUR CONTENT BELOW HERE --> | <!-- PLEASE ADD YOUR CONTENT BELOW HERE --> | ||
| - | Hi | + | Hi Lakshmi, |
| - | Here is your very own Proteopedia Page | + | Here is your very own Proteopedia Page for pdb code: 1PUF. |
| - | + | Your presentation is scheduled for: June 4. | |
| - | + | ||
| - | + | ||
Have Fun! | Have Fun! | ||
Greg Buhrman | Greg Buhrman | ||
| + | [[Image:1puf.png|left|200px]] | ||
| + | |||
| + | <!-- | ||
| + | The line below this paragraph, containing "STRUCTURE_1puf", creates the "Structure Box" on the page. | ||
| + | You may change the PDB parameter (which sets the PDB file loaded into the applet) | ||
| + | or the SCENE parameter (which sets the initial scene displayed when the page is loaded), | ||
| + | or leave the SCENE parameter empty for the default display. | ||
| + | --> | ||
| + | {{STRUCTURE_1puf| PDB=1puf | SCENE= }} | ||
| + | |||
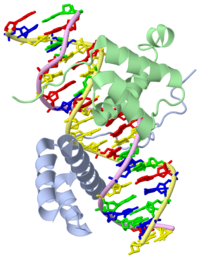
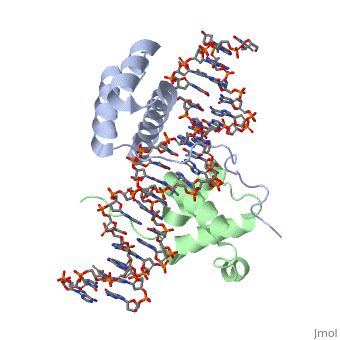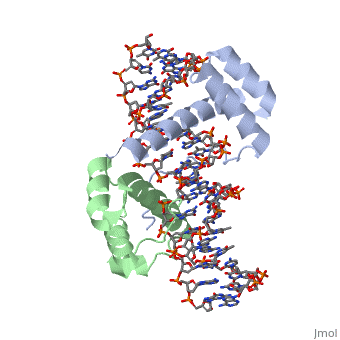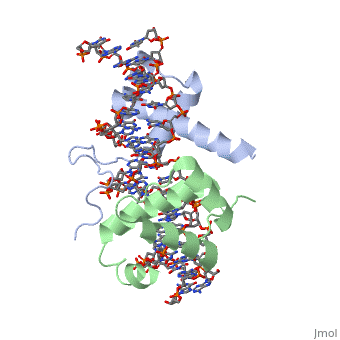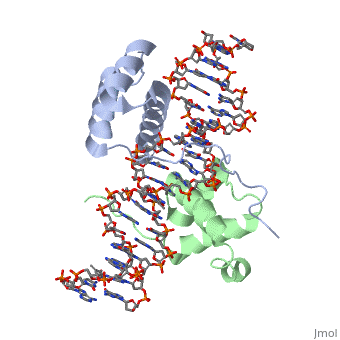
| + | ===Crystal Structure of HoxA9 and Pbx1 homeodomains bound to DNA=== | ||
| + | |||
| + | |||
| + | <!-- | ||
| + | The line below this paragraph, {{ABSTRACT_PUBMED_12923056}}, adds the Publication Abstract to the page | ||
| + | (as it appears on PubMed at http://www.pubmed.gov), where 12923056 is the PubMed ID number. | ||
| + | --> | ||
| + | {{ABSTRACT_PUBMED_12923056}} | ||
| + | |||
| + | ==About this Structure== | ||
| + | [[1puf]] is a 4 chain structure with sequence from [http://en.wikipedia.org/wiki/Homo_sapiens Homo sapiens] and [http://en.wikipedia.org/wiki/Mus_musculus Mus musculus]. Full crystallographic information is available from [http://oca.weizmann.ac.il/oca-bin/ocashort?id=1PUF OCA]. | ||
| + | |||
| + | ==See Also== | ||
| + | *[[Tomato aspermy virus protein 2b Suppression of RNA Silencing]] | ||
| + | *[[User:Wayne Decatur/Tomato aspermy virus protein 2b Suppression of RNA Silencing]] | ||
| + | |||
| + | ==Reference== | ||
| + | <ref group="xtra">PMID:12923056</ref><references group="xtra"/> | ||
| + | [[Category: Homo sapiens]] | ||
| + | [[Category: Mus musculus]] | ||
| + | [[Category: Laronde-Leblanc, N A.]] | ||
| + | [[Category: Wolberger, C.]] | ||
| + | [[Category: Homeodomain interaction]] | ||
| + | [[Category: Homeodomian]] | ||
| + | [[Category: Hox hexapeptide]] | ||
| + | [[Category: Protein-dna complex]] | ||
| + | [[Category: Tale homeodomain]] | ||
| + | [[Category: Transcription/dna complex]] | ||
Current revision
| This Sandbox is Reserved from 05/22/2012, through 07/22/2012 for use in the course "BIOL 414" taught by Greg Buhrman at the North Carolina State University, Raleigh, NC USA. This reservation includes Sandbox Reserved 551 through Sandbox Reserved 590. |
To get started:
More help: Help:Editing |
Hi Lakshmi, Here is your very own Proteopedia Page for pdb code: 1PUF. Your presentation is scheduled for: June 4.
Have Fun!
Greg Buhrman
Contents |
Crystal Structure of HoxA9 and Pbx1 homeodomains bound to DNA
The HOX/HOM superfamily of homeodomain proteins controls cell fate and segmental embryonic patterning by a mechanism that is conserved in all metazoans. The linear arrangement of the Hox genes on the chromosome correlates with the spatial distribution of HOX protein expression along the anterior-posterior axis of the embryo. Most HOX proteins bind DNA cooperatively with members of the PBC family of TALE-type homeodomain proteins, which includes human Pbx1. Cooperative DNA binding between HOX and PBC proteins requires a residue N-terminal to the HOX homeodomain termed the hexapeptide, which differs significantly in sequence between anterior- and posterior-regulating HOX proteins. We report here the 1.9-A-resolution structure of a posterior HOX protein, HoxA9, complexed with Pbx1 and DNA, which reveals that the posterior Hox hexapeptide adopts an altered conformation as compared with that seen in previously determined anterior HOX/PBC structures. The additional nonspecific interactions and altered DNA conformation in this structure account for the stronger DNA-binding affinity and altered specificity observed for posterior HOX proteins when compared with anterior HOX proteins. DNA-binding studies of wild-type and mutant HoxA9 and HoxB1 show residues in the N-terminal arm of the homeodomains are critical for proper DNA sequence recognition despite lack of direct contact by these residues to the DNA bases. These results help shed light on the mechanism of transcriptional regulation by HOX proteins and show how DNA-binding proteins may use indirect contacts to determine sequence specificity.
Structure of HoxA9 and Pbx1 bound to DNA: Hox hexapeptide and DNA recognition anterior to posterior., LaRonde-LeBlanc NA, Wolberger C, Genes Dev. 2003 Aug 15;17(16):2060-72. PMID:12923056
From MEDLINE®/PubMed®, a database of the U.S. National Library of Medicine.
About this Structure
1puf is a 4 chain structure with sequence from Homo sapiens and Mus musculus. Full crystallographic information is available from OCA.
See Also
- Tomato aspermy virus protein 2b Suppression of RNA Silencing
- User:Wayne Decatur/Tomato aspermy virus protein 2b Suppression of RNA Silencing
Reference
- LaRonde-LeBlanc NA, Wolberger C. Structure of HoxA9 and Pbx1 bound to DNA: Hox hexapeptide and DNA recognition anterior to posterior. Genes Dev. 2003 Aug 15;17(16):2060-72. PMID:12923056 doi:http://dx.doi.org/10.1101/gad.1103303