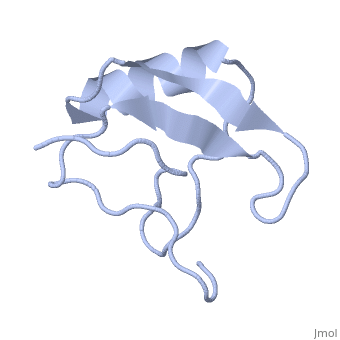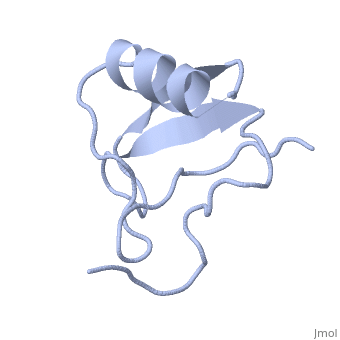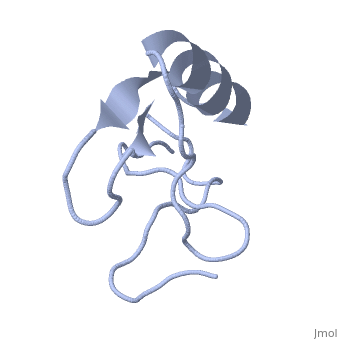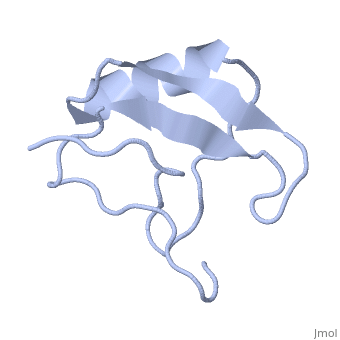
Scorpion toxin
From Proteopedia
| Line 1: | Line 1: | ||
| + | <StructureSection load='1lqh' size="400" color="" frame="true" spin="on" Scene= align="right" caption='Scorpion toxin, [[1lqh]]' > | ||
[[Image:1lqh.png|left|200px|thumb|Scorpion toxin [[1lqh]]]] | [[Image:1lqh.png|left|200px|thumb|Scorpion toxin [[1lqh]]]] | ||
| - | {{STRUCTURE_1lqh| PDB=1lqh | SIZE=400| SCENE= |right|CAPTION=Scorpion toxin [[1lqh]] }} | ||
| - | |||
| - | <font color='red'><b>Under construction!</b></font> <br /> | ||
==Overview== | ==Overview== | ||
Scorpion toxins are 61- to 76-residue long proteins that modulate the gating properties of voltage-gated sodium channels. According to their mode of action and binding properties,scorpion toxins are divided into two major classes, alpha- and beta- toxins. | Scorpion toxins are 61- to 76-residue long proteins that modulate the gating properties of voltage-gated sodium channels. According to their mode of action and binding properties,scorpion toxins are divided into two major classes, alpha- and beta- toxins. | ||
| Line 16: | Line 14: | ||
NMR studies of LqhαIT with a peptide derived from part of neurotoxin receptor site-3 (D4S3-S4) identified residues <scene name='1lqh/Nmr/1'>Lys8, Asn9, Cys12, Val13, Arg18, Trp38, Ala39 and Arg58</scene> of LqhαIT as participating in binding to that extra-cellular part of the sodium channel (3). | NMR studies of LqhαIT with a peptide derived from part of neurotoxin receptor site-3 (D4S3-S4) identified residues <scene name='1lqh/Nmr/1'>Lys8, Asn9, Cys12, Val13, Arg18, Trp38, Ala39 and Arg58</scene> of LqhαIT as participating in binding to that extra-cellular part of the sodium channel (3). | ||
Both methods led to the identification of two regions on the surface of LqhαIT that participate in binding of the toxin to neurotoxin receptor site-3 on sodium channels. | Both methods led to the identification of two regions on the surface of LqhαIT that participate in binding of the toxin to neurotoxin receptor site-3 on sodium channels. | ||
| - | + | </StructureSection> | |
==References== | ==References== | ||
(1) Tugarinov, V., Kustanovich, I., Zilberberg, N., Gurevitz, M. Anglister, J. Solution structures of a highly insecticidal recombinant scorpion alpha-toxin and a mutant with increased activity. Biochemistry 36, 2414-24 (1997). | (1) Tugarinov, V., Kustanovich, I., Zilberberg, N., Gurevitz, M. Anglister, J. Solution structures of a highly insecticidal recombinant scorpion alpha-toxin and a mutant with increased activity. Biochemistry 36, 2414-24 (1997). | ||
Revision as of 12:46, 11 July 2012
| |||||||||||
References
(1) Tugarinov, V., Kustanovich, I., Zilberberg, N., Gurevitz, M. Anglister, J. Solution structures of a highly insecticidal recombinant scorpion alpha-toxin and a mutant with increased activity. Biochemistry 36, 2414-24 (1997). PMID: 9054546
(2) Karbat, I., Frolow, F., Froy, O., Gilles, N., Cohen, L., Turkov, M., Gordon, D. Gurevitz, M. Molecular basis of the high insecticidal potency of scorpion alpha-toxins. J. Biol. Chem. 279, 31679-86 (2004). PMID: 15133045
(3) Schnur, E., Turkov, M., Kahn, R., Gordon, D., Gurevitz, M., Anglister, J. NMR Analysis of interaction of Lqh(alpha)IT scorpion toxin with a peptide corresponding to the D4/S3-S4 loop of insect para voltage-gated sodium channel. Biochemistry 47(3), 911-21 (2008).
PMID: 18154318
Created with the participation of Eran Hodis, Einat Schnur, Jaime Prilusky