We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
Titin
From Proteopedia
(Difference between revisions)
| Line 1: | Line 1: | ||
| - | + | <StructureSection load="2a38" size="300" color="" frame="true" spin="on" Scene ="" align="right" caption=""> | |
| - | + | ||
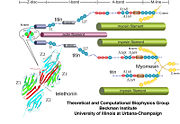
| - | + | [[Titin]] (TTN) is the largest known protein. The human TTN contains 34,350 residues. It is responsible for the passive elasticity of muscle. It has 244 domains connected by unstructured regions. The domains unfold when TTN is stretched. Additional details in [[Titin Structure & Function]]. | |
| - | [[Titin]] (TTN) is the largest known protein. The human TTN contains 34,350 residues. It is responsible for the passive elasticity of muscle. It has 244 domains connected by unstructured regions. The domains unfold when TTN is stretched. | + | |
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
[[Image:2a38 bio assembly1.jpg|225px]] | [[Image:2a38 bio assembly1.jpg|225px]] | ||
| Line 44: | Line 37: | ||
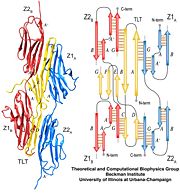
As seen in the image (Z1Z2 / Telethonin complex), the major force enduring component of this complex is an elaborate intermolecular hydrogen bonding network formed across <scene name='2a38/Test/2'>β-strand</scene> among telethonin and Z1Z2 domains, and not intramolecularly among termini β-strands of individual Z1 or Z2 domains. This shift to a stronger force enduring interface reduces the possibility of unraveling the individual Ig-domains, thus stabilizing the complex. This demonstrates how <scene name='2a38/Test/2'>β-strand</scene> cross-linking via [http://en.wikipedia.org/wiki/Hydrogen_bonds hydrogen bonds] serves as an important mechanism. It functions as a molecular adhesive, increasing the ability of protein complexes to resist against mechanical stress. | As seen in the image (Z1Z2 / Telethonin complex), the major force enduring component of this complex is an elaborate intermolecular hydrogen bonding network formed across <scene name='2a38/Test/2'>β-strand</scene> among telethonin and Z1Z2 domains, and not intramolecularly among termini β-strands of individual Z1 or Z2 domains. This shift to a stronger force enduring interface reduces the possibility of unraveling the individual Ig-domains, thus stabilizing the complex. This demonstrates how <scene name='2a38/Test/2'>β-strand</scene> cross-linking via [http://en.wikipedia.org/wiki/Hydrogen_bonds hydrogen bonds] serves as an important mechanism. It functions as a molecular adhesive, increasing the ability of protein complexes to resist against mechanical stress. | ||
| - | |||
| - | </StructureSection> | ||
== 3D Structures of Titin == | == 3D Structures of Titin == | ||
| Line 78: | Line 69: | ||
==See Also== | ==See Also== | ||
http://www.biophysik.physik.uni-muenchen.de/movies/movies-and-animations | http://www.biophysik.physik.uni-muenchen.de/movies/movies-and-animations | ||
| + | </StructureSection> | ||
| + | |||
==References== | ==References== | ||
Revision as of 11:32, 7 June 2012
| |||||||||||
References
- http://www.ncbi.nlm.nih.gov:80/pmc/articles/PMC1948054/?tool=pmcentrez
- http://www.ks.uiuc.edu/Research/z1z2/
- http://www.ks.uiuc.edu/Research/telethonin/
- http://de.wikipedia.org/wiki/Titin
Created with the participation of Anton Schmidt, Wolfgang Hermann.
Proteopedia Page Contributors and Editors (what is this?)
Michal Harel, Alexander Berchansky, David Canner, Jaime Prilusky
Categories: Topic Page | Homo sapiens | Demirel, M. | Marino, M. | Mayans, O. | Muhle-Goll, C. | Svergun, D. | Titin | Z1z2