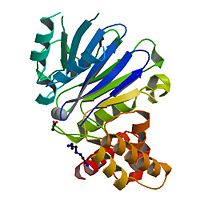
Leishmania infantum Glyoxalase II
From Proteopedia
(Difference between revisions)
(New page: <StructureSection load="2p18" size="400" color="" frame="true" spin="on" Scene= align="right" caption= > 200px <!-- The line below this paragraph, containing "S...) |
|||
| Line 3: | Line 3: | ||
[[Image:2p18.jpg|left|200px]] | [[Image:2p18.jpg|left|200px]] | ||
| - | + | '''''Leishmania infantum'' Glyoxalase II''' | |
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | '''<FONT COLOR="#F535AA">''Leishmania infantum'' Glyoxalase II</FONT>''' | ||
| - | + | ==Overview== | |
| - | + | ||
| - | == | + | |
The glyoxalase pathway catalyzes the formation of D-lactate from methylglyoxal, a toxic byproduct of glycolysis. In trypanosomatids, trypanothione replaces glutathione in this pathway, making it a potential drug target, since its selective inhibition might increase methylglyoxal concentration in the parasites. | The glyoxalase pathway catalyzes the formation of D-lactate from methylglyoxal, a toxic byproduct of glycolysis. In trypanosomatids, trypanothione replaces glutathione in this pathway, making it a potential drug target, since its selective inhibition might increase methylglyoxal concentration in the parasites. | ||
Evolutionary analysis shows that trypanosomatid ''L. infantum'' glyoxalase II diverged early from eukaryotic enzymes, being unrelated to prokaryotic proteins. This enzyme shows absolute specificity towards trypanothione, making it an exceptional model to understand the molecular basis of trypanothione binding and specificity. | Evolutionary analysis shows that trypanosomatid ''L. infantum'' glyoxalase II diverged early from eukaryotic enzymes, being unrelated to prokaryotic proteins. This enzyme shows absolute specificity towards trypanothione, making it an exceptional model to understand the molecular basis of trypanothione binding and specificity. | ||
| - | == | + | ==''Leishmania infantum''== |
Leimaniasis is a disease caused by flagellated protozoa, belonging to the Trypanosomatids family. The Trypanosomatids are the etiological agents of several human and animal diseases, widespread in the third world and in the Mediterranean basin. It is estimated that 12 million people in the World are infected with one of this disease forms and that 2 million new cases are detected per year in 88 countries. Emergent target populations for leishmaniasis are HIV patients and people following immunosupressor therapies. No curative drugs or vaccines currently exist and actual therapeutic approaches are becoming limited given their undesirable side effects and the evolution of resistant forms of trypanosomatids. | Leimaniasis is a disease caused by flagellated protozoa, belonging to the Trypanosomatids family. The Trypanosomatids are the etiological agents of several human and animal diseases, widespread in the third world and in the Mediterranean basin. It is estimated that 12 million people in the World are infected with one of this disease forms and that 2 million new cases are detected per year in 88 countries. Emergent target populations for leishmaniasis are HIV patients and people following immunosupressor therapies. No curative drugs or vaccines currently exist and actual therapeutic approaches are becoming limited given their undesirable side effects and the evolution of resistant forms of trypanosomatids. | ||
| Line 27: | Line 19: | ||
Trypanosomatids have 2 unique characteristics: the first is the compartimentation of glycolysis in the glycosome; and the second is the functional replacement of glutathione by trypanothione and, consequently, the replacement of the gluthatione-dependent enzymes by trypanothione-dependent ones. | Trypanosomatids have 2 unique characteristics: the first is the compartimentation of glycolysis in the glycosome; and the second is the functional replacement of glutathione by trypanothione and, consequently, the replacement of the gluthatione-dependent enzymes by trypanothione-dependent ones. | ||
| - | == | + | ==Trypanothione== |
Trypanothione is a compound produced by Trypanosomatids, formed by two glutathione molecules bound by a spermidine molecule. | Trypanothione is a compound produced by Trypanosomatids, formed by two glutathione molecules bound by a spermidine molecule. | ||
| - | == | + | ==The Glyoxalase Pathway== |
The glyoxalase system is the main detoxification pathway of methylglyoxal, which is a toxic and mutagenic byproduct of glycolysis. | The glyoxalase system is the main detoxification pathway of methylglyoxal, which is a toxic and mutagenic byproduct of glycolysis. | ||
| Line 36: | Line 28: | ||
In ''Leishmania infantum'', the glyoxalase I has activity with glutathione, although it preferentially uses trypanothione. Glyoxalase II is specific to S-D-lactoyl-trypanothione, being an outstanding model to study trypanothione specificity. | In ''Leishmania infantum'', the glyoxalase I has activity with glutathione, although it preferentially uses trypanothione. Glyoxalase II is specific to S-D-lactoyl-trypanothione, being an outstanding model to study trypanothione specificity. | ||
| - | == | + | ==The ''L. infantum'' Glyoxalase II Structure== |
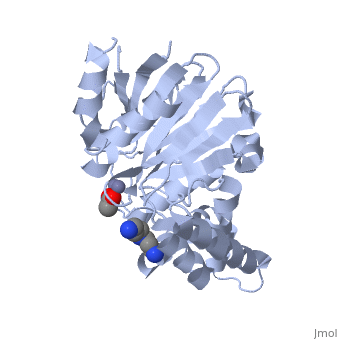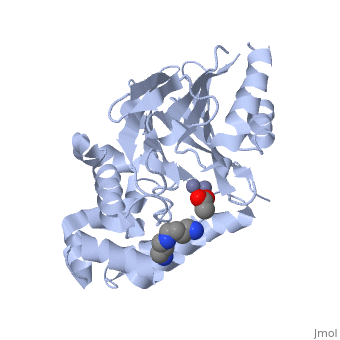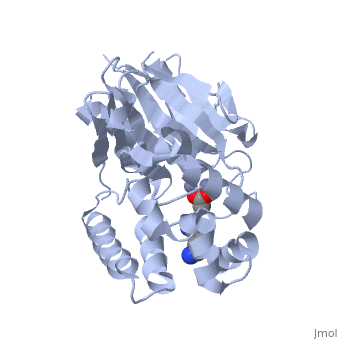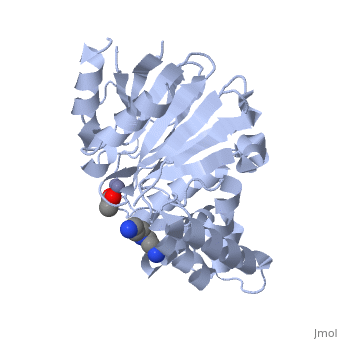
The crystal structure of ''Leishmania infantum'' glyoxalase II (2P18) is the first structure of this enzyme from trypanosomatids. It is a 295 amino acid metalloprotein. The overall structure of Leishmania infantum glyoxalase II is very similar to its human counterpart. Like the human glx II, it is a monomer arranged in two domains and its active site is very conserved. However it shows important differences at the substrate binding site. | The crystal structure of ''Leishmania infantum'' glyoxalase II (2P18) is the first structure of this enzyme from trypanosomatids. It is a 295 amino acid metalloprotein. The overall structure of Leishmania infantum glyoxalase II is very similar to its human counterpart. Like the human glx II, it is a monomer arranged in two domains and its active site is very conserved. However it shows important differences at the substrate binding site. | ||
Full crystallographic information is available from [http://oca.weizmann.ac.il/oca-bin/ocashort?id=2P18 OCA]. | Full crystallographic information is available from [http://oca.weizmann.ac.il/oca-bin/ocashort?id=2P18 OCA]. | ||
| - | == | + | ==Reference== |
Catalysis and structural properties of Leishmania infantum glyoxalase II: trypanothione specificity and phylogeny., Sousa Silva M, Barata L, Ferreira AE, Romao S, Tomas AM, Ponces Freire A, Cordeiro C, Biochemistry. 2008 Jan 8;47(1):195-204. Epub 2007 Dec 6. PMID:[http://www.ncbi.nlm.nih.gov/pubmed/18052346 18052346] | Catalysis and structural properties of Leishmania infantum glyoxalase II: trypanothione specificity and phylogeny., Sousa Silva M, Barata L, Ferreira AE, Romao S, Tomas AM, Ponces Freire A, Cordeiro C, Biochemistry. 2008 Jan 8;47(1):195-204. Epub 2007 Dec 6. PMID:[http://www.ncbi.nlm.nih.gov/pubmed/18052346 18052346] | ||
[[Category: Hydroxyacylglutathione hydrolase]] | [[Category: Hydroxyacylglutathione hydrolase]] | ||
Revision as of 12:45, 11 June 2012
| |||||||||||