This old version of Proteopedia is provided for student assignments while the new version is undergoing repairs. Content and edits done in this old version of Proteopedia after March 1, 2026 will eventually be lost when it is retired in about June of 2026.
Apply for new accounts at the new Proteopedia. Your logins will work in both the old and new versions.
2p1q
From Proteopedia
m (Protected "2p1q" [edit=sysop:move=sysop]) |
|||
| Line 20: | Line 20: | ||
==About this Structure== | ==About this Structure== | ||
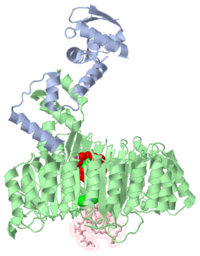
[[2p1q]] is a 3 chain structure with sequence from [http://en.wikipedia.org/wiki/Arabidopsis_thaliana Arabidopsis thaliana]. The February 2009 RCSB PDB [http://pdb.rcsb.org/pdb/static.do?p=education_discussion/molecule_of_the_month/index.html Molecule of the Month] feature on ''Auxin and TIR1 Ubiquitin Ligase'' by David Goodsell is [http://dx.doi.org/10.2210/rcsb_pdb/mom_2009_2 10.2210/rcsb_pdb/mom_2009_2]. Full crystallographic information is available from [http://oca.weizmann.ac.il/oca-bin/ocashort?id=2P1Q OCA]. | [[2p1q]] is a 3 chain structure with sequence from [http://en.wikipedia.org/wiki/Arabidopsis_thaliana Arabidopsis thaliana]. The February 2009 RCSB PDB [http://pdb.rcsb.org/pdb/static.do?p=education_discussion/molecule_of_the_month/index.html Molecule of the Month] feature on ''Auxin and TIR1 Ubiquitin Ligase'' by David Goodsell is [http://dx.doi.org/10.2210/rcsb_pdb/mom_2009_2 10.2210/rcsb_pdb/mom_2009_2]. Full crystallographic information is available from [http://oca.weizmann.ac.il/oca-bin/ocashort?id=2P1Q OCA]. | ||
| + | |||
| + | ==See Also== | ||
| + | *[[Herbicide 2%2C4-D bound to TIR1 Ubiquitin Ligase|Herbicide 2%2C4-D bound to TIR1 Ubiquitin Ligase]] | ||
| + | *[[TIR1 ubiquitin ligase|TIR1 ubiquitin ligase]] | ||
==Reference== | ==Reference== | ||
Revision as of 07:21, 13 June 2012
Contents |
Mechanism of Auxin Perception by the TIR1 ubiquitin ligase
Auxin is a pivotal plant hormone that controls many aspects of plant growth and development. Perceived by a small family of F-box proteins including transport inhibitor response 1 (TIR1), auxin regulates gene expression by promoting SCF ubiquitin-ligase-catalysed degradation of the Aux/IAA transcription repressors, but how the TIR1 F-box protein senses and becomes activated by auxin remains unclear. Here we present the crystal structures of the Arabidopsis TIR1-ASK1 complex, free and in complexes with three different auxin compounds and an Aux/IAA substrate peptide. These structures show that the leucine-rich repeat domain of TIR1 contains an unexpected inositol hexakisphosphate co-factor and recognizes auxin and the Aux/IAA polypeptide substrate through a single surface pocket. Anchored to the base of the TIR1 pocket, auxin binds to a partially promiscuous site, which can also accommodate various auxin analogues. Docked on top of auxin, the Aux/IAA substrate peptide occupies the rest of the TIR1 pocket and completely encloses the hormone-binding site. By filling in a hydrophobic cavity at the protein interface, auxin enhances the TIR1-substrate interactions by acting as a 'molecular glue'. Our results establish the first structural model of a plant hormone receptor.
Mechanism of auxin perception by the TIR1 ubiquitin ligase., Tan X, Calderon-Villalobos LI, Sharon M, Zheng C, Robinson CV, Estelle M, Zheng N, Nature. 2007 Apr 5;446(7136):640-5. PMID:17410169
From MEDLINE®/PubMed®, a database of the U.S. National Library of Medicine.
About this Structure
2p1q is a 3 chain structure with sequence from Arabidopsis thaliana. The February 2009 RCSB PDB Molecule of the Month feature on Auxin and TIR1 Ubiquitin Ligase by David Goodsell is 10.2210/rcsb_pdb/mom_2009_2. Full crystallographic information is available from OCA.
See Also
Reference
- Tan X, Calderon-Villalobos LI, Sharon M, Zheng C, Robinson CV, Estelle M, Zheng N. Mechanism of auxin perception by the TIR1 ubiquitin ligase. Nature. 2007 Apr 5;446(7136):640-5. PMID:17410169 doi:10.1038/nature05731