This old version of Proteopedia is provided for student assignments while the new version is undergoing repairs. Content and edits done in this old version of Proteopedia after March 1, 2026 will eventually be lost when it is retired in about June of 2026.
Apply for new accounts at the new Proteopedia. Your logins will work in both the old and new versions.
Quinone reductase
From Proteopedia
| Line 1: | Line 1: | ||
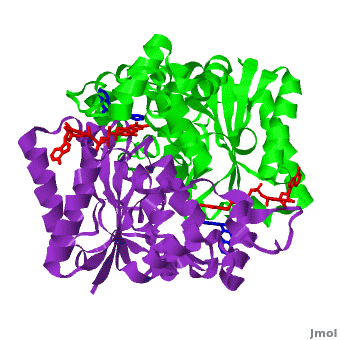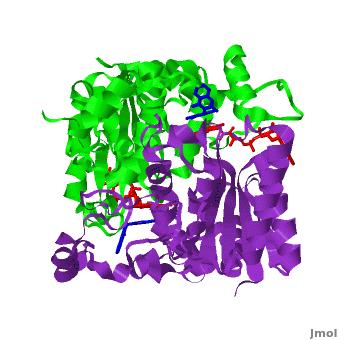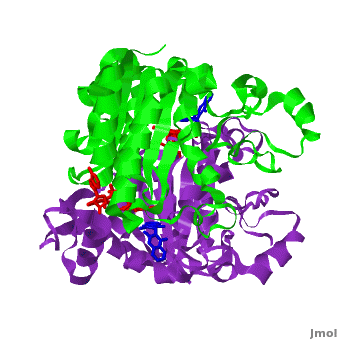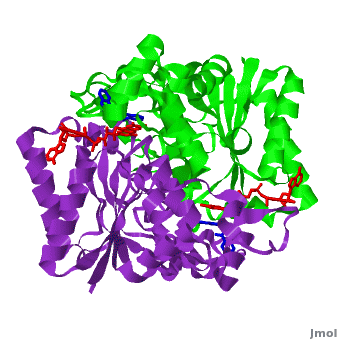
[[Image:2f1o1.png|left|200px|thumb|Crystal Structure of NADH quinone oxidoreductase (NQO1) with inhibitor dicoumarol [[2f1o]]]] | [[Image:2f1o1.png|left|200px|thumb|Crystal Structure of NADH quinone oxidoreductase (NQO1) with inhibitor dicoumarol [[2f1o]]]] | ||
| - | {{STRUCTURE_2f1o| PDB=2f1o | SIZE=400| SCENE=2f1o/Com_view/2 |right|CAPTION=NADH quinone oxidoreductase (NQO1) with inhibitor dicoumarol [[2f1o]] }} | + | {{STRUCTURE_2f1o| PDB=2f1o | SIZE=400| SCENE=2f1o/Com_view/2 |right|CAPTION=NADH quinone oxidoreductase (NQO1) dimer with FAD (red) and inhibitor dicoumarol (blue) [[2f1o]] }} |
Revision as of 07:53, 14 November 2012
Quinone reductase type 1 (QR1) reduces quinines to the non-toxic hydroquinone. Quinone reductase type 2 (QR2) catalyzes the reduction of adrenochrome. The sulfide-quinone reductase (SQR) reduces sulfide and thus provides electrons for phototropic processes in bacteria. NADPH-quinone reductase (NQR) catalyzes the reduction of quinone to semiquinone. The images at the left and at the right correspond to one representative Quinone reductase, i.e. the crystal structure of NADH quinone oxidoreductase (NQO1) with inhibitor dicoumarol (2f1o).
Contents |
NADH Quinone oxidoreductase type 1 (NQO1) in complex with its potent inhibitor dicoumarol
NAD(P)H quinone oxidoreductase 1 (NQO1, EC 1.6.5.2) is a ubiquitous flavoenzyme that catalyzes two electron reduction of quinones to hydroquinones utilizing NAD(P)H as an electron donor. NQO1 is a homo-dimer that functions via a “ping pong” mechanism. NAD(P)H binds to NQO1, reduces the FAD co-factor and is then released, allowing the quinone substrate to bind the enzyme and to be reduced. The NAD(P)H and the quinone binding sites of NQO1 have a significant overlap, thus providing a molecular basis for this “ping pong” mechanism. Certain coumarins, flavones and the reactive dye cibacron blue are competitive inhibitors of NQO1 activity, which compete with NAD(P)H for binding to NQO1. Dicoumarol (3-3’–methylene-bis (4-hydroxycoumarin)), is the most potent competitive inhibitor of NQO1. Dicoumarol competes with NAD(P)H for binding to NQO1 and prevents the electron transfer to FAD. In addition to its role in the detoxification of quinones, NQO1 is also a 20S proteasome-associated protein that plays an important role in the stability of the tumor suppressor p53 and several other short-lived proteins including p73α and ornithine decarboxylase (ODC, i.e. 7odc). NQO1 binds and stabilizes p53, protecting p53 from ubiquitin-independent 20S proteasomal degradation. Dicoumarol and several other inhibitors of NQO1 activity, which compete with NADH for binding to NQO1, disrupt the binding of NQO1 to p53 and induce ubiquitin-independent p53 degradation.
| |||||||||||
3D Structures of Quinone reductase
Updated June 2012
Quinone reductase type 1
3jsx – hQR1 + coumarine derivative
2f1o – hQR1 + dicoumarol
1kbo, 1kbq – hQR1 + indole derivative
Quinone reductase type 2
3fw1, 1qr2 – hQR2 - human
3o2n, 3g5m, 3gam – hQR2 + PET agent
3ovm, 3owh, 3owx – hQR2 + carbamate derivative
3ox1, 2qx4, 2qx6, 2qx8, 2qx9, 2qwx – hQR2 + acetamide derivative
3ox2 - hQR2 + indole derivative
3ox3 - hQR2 + carboxamide derivative
3o73 – hQR2 + indolequinone
2qmy – hQR2 + adrenochrome
2qr2 – hQR2 + menadione
2qmz – hQR2 + dopamine
1sg0 – hQR2 + resveratol
3nhu, 3nhs, 3nhr, 3nhp, 3nhl, 3nhk, 3nhj, 3nhf, 3nfr, 3nhw, 3nhy, 3uxe, 3uxh - hQR2 + quinoline derivative
3te7, 3tem, 3tzb – hQR2 + acridine derivative
2bzs, 1xi2, 1zx1 – hQR2 + anti-cancer prodrug
Sulfide-quinone reductase
3hyv – AaSQR – Aquifex aeolicus
3hyw – AaSQR + decylubiquinone
3hyx – AaSQR + Aurachin C
NADPH-quinone reductase
3ha2 – NQR – Pediococcus pentosaceus
1dxq, 1d4a - hNQR
1yb5 – hNQR + NADP
1gg5, 1h66, 1h69, 1qbg - hNQR + anti-cancer prodrug
1dxo - hNQR + quinone derivative
1qrd – QR + bicarbon blue + duroquinone - rat
References
- Faig M, Bianchet MA, Talalay P, Chen S, Winski S, Ross D, Amzel LM. Structures of recombinant human and mouse NAD(P)H:quinone oxidoreductases: species comparison and structural changes with substrate binding and release. Proc Natl Acad Sci U S A. 2000 Mar 28;97(7):3177-82. PMID:10706635 doi:http://dx.doi.org/10.1073/pnas.050585797
- Asher G, Dym O, Tsvetkov P, Adler J, Shaul Y. The crystal structure of NAD(P)H quinone oxidoreductase 1 in complex with its potent inhibitor dicoumarol. Biochemistry. 2006 May 23;45(20):6372-8. PMID:16700548 doi:10.1021/bi0600087